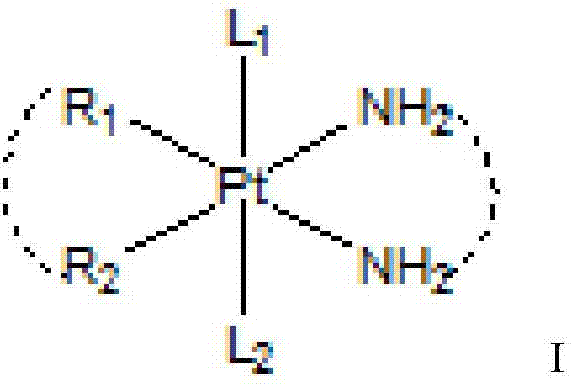
Platinum compound with anti-tumor activity as well as preparation method and application
A complex and platinum-based technology, applied in anti-cancer and anti-tumor applications, new tetravalent platinum complexes and their preparation, can solve the problem of less combined work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
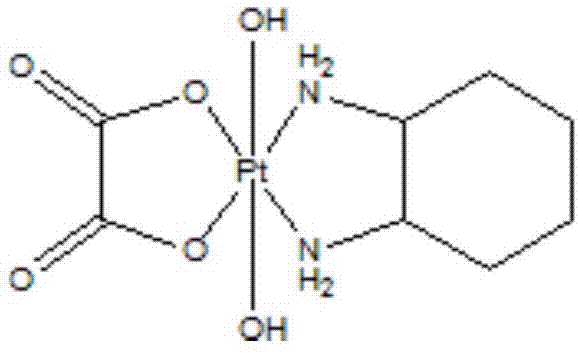
[0019] The preparation of embodiment 1 hydroxy oxaliplatin (IV)
[0020]
[0021] Add 3.0 g of oxaliplatin and 70 mL of distilled water into the flask, stir to disperse them, slowly add 130 mL of 30% hydrogen peroxide dropwise into the reaction system, raise the temperature to 65°C and stir for 3 hours. The reaction was stopped, placed under cooling for crystallization for 12 hours, filtered to obtain a yellow solid, added an appropriate amount of water, heated to dissolve it, cooled and allowed to stand for crystallization for 12 hours, and filtered to obtain white crystals (2.5 g).
Embodiment 2
[0022] The preparation of the monohydroxy oxaliplatin (IV) that embodiment 2 carboxylic acid group replaces
[0023]
[0024] Add 1.6 g of the compound prepared in Example 1 and 0.79 g of hexanoic anhydride to a round bottom flask, add 70 mL of anhydrous DMSO under nitrogen protection, stir and react at 35°C for 4 days, evaporate DMSO under reduced pressure, add acetone, and precipitate The white solid powder was suction filtered and washed 3 times with acetone to obtain a white solid product (1.5 g).
Embodiment 3
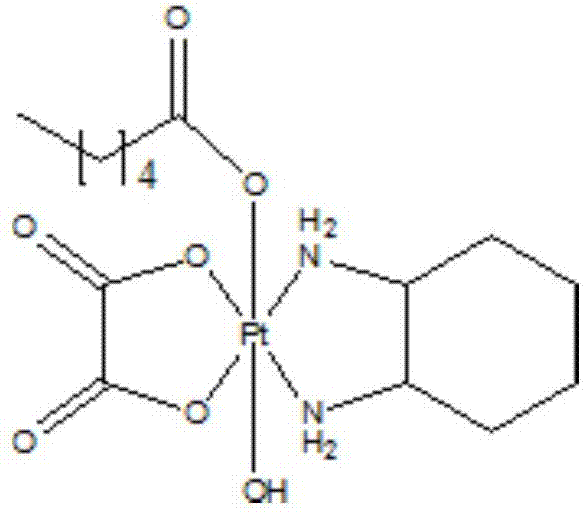
[0025] Embodiment 3 target product is synthesized
[0026]
[0027] Add 0.01 mol of the tetravalent platinum intermediate prepared in Example 2 and 0.02 mol of galloyl chloride protected by whole benzyl groups into the flask, protect with nitrogen and add anhydrous acetone, stir at room temperature for 12 to 36 hours, and stop the reaction. Acetone was removed by concentration, and the product was obtained by column chromatography. Dissolve the product in an appropriate amount of anhydrous dichloromethane, stir at -78°C for 15 minutes, slowly add 1M boron trichloride / dichloromethane solution dropwise to the reaction solution, continue stirring for 2 hours, and stop the reaction. The solvent was distilled off under reduced pressure, and the residual solid was washed three times with petroleum ether, and separated by column chromatography to obtain the target product, which was designated as compound 1.
[0028] The product is a light yellow solid; 1 H NMR (400MHz, DMSO-d ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com