High performance liquid chromatography analysis method and detection method for impurities in Telmisartan medicine
A technology of high performance liquid chromatography and analysis method, which is applied in the field of high performance liquid chromatography analysis method and the detection of impurities in telmisartan medicine, can solve the problems of difficult detection process, time-consuming reagent dosage, and high detection cost, and achieves shortened time. Analysis time, simple operation, and the effect of changing many operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
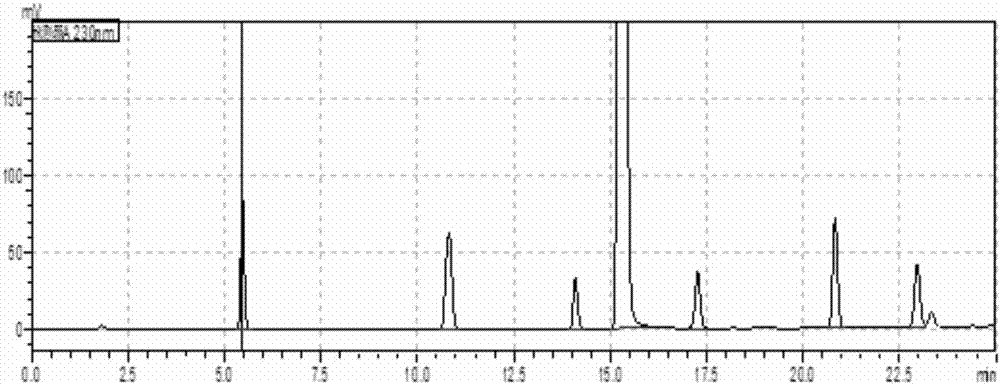
Examples
preparation example 1
[0047] Accurately weigh telmisartan and impurity A, impurity B, impurity C, impurity E, impurity F, impurity G, impurity H, impurity I, add 0.005mol / L sodium hydroxide methanol solution to prepare a solution, wherein , the mass concentration of telmisartan is 0.5 mg / mL; the respective mass concentrations of impurity A, impurity B, impurity C, impurity E, impurity F, impurity G, impurity H and impurity I in the solution are 5 μg / mL respectively.
Embodiment 1
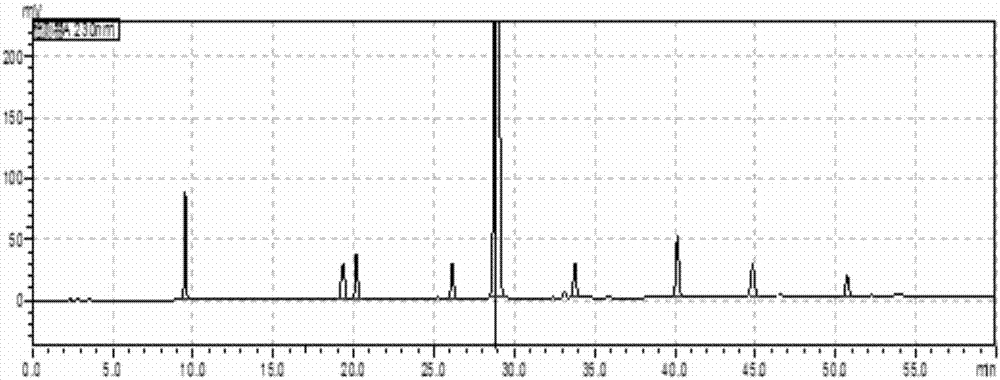
[0049] The solution in the preparation example 1 is analyzed with high performance liquid chromatography, and the record chromatogram, the chromatographic condition of described high performance liquid chromatography is as follows:
[0050] Chromatographic column: 150mm×4.6μm 5μm C18, column temperature 35℃
[0051] Wavelength: 230nm
[0052] Injection volume: 10μL
[0053] Flow rate: 1.0mL / min
[0054] The first mobile phase: add 2.0g potassium dihydrogen phosphate and 3.8g sodium n-pentanesulfonate monohydrate to 1L water, adjust the pH value to 3.5 with phosphoric acid;
[0055] The second mobile phase: the volume ratio of methanol and acetonitrile is 50:50;
[0056] In the gradient elution, the total volume of the mobile phase is 100%, and the conditions of the gradient elution are set according to time as shown in Table 1:
[0057] Table 1
[0058] time (min)
The first mobile phase (%)
Second mobile phase (%)
0-10
50
50
10-30
50...
Embodiment 2
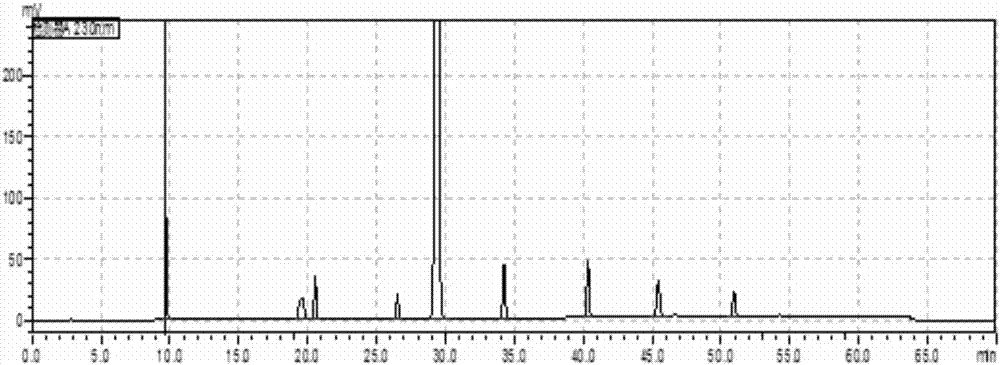
[0065] The solution in the preparation example 1 is analyzed with high performance liquid chromatography, and the record chromatogram, the chromatographic condition of described high performance liquid chromatography is as follows:
[0066] Chromatographic column: 150mm×4.6μm 5μm C18, column temperature 35℃
[0067] Wavelength: 230nm
[0068] Injection volume: 10μL
[0069] Flow rate: 1.0mL / min
[0070] The first mobile phase: add 2.0g potassium dihydrogen phosphate and 3.8g sodium n-pentanesulfonate monohydrate to 1L water, adjust the pH value to 3.0 with phosphoric acid;
[0071] The second mobile phase: the volume ratio of methanol and acetonitrile is 50:50;
[0072] In the gradient elution, the total volume of the mobile phase is taken as 100%, and the conditions of the gradient elution are set as shown in Table 3 by time.
[0073] table 3
[0074]
[0075] The obtained chromatogram is as figure 2 shown, seen, figure 2 9 absorption peaks were obtained in the so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com