Preparation method for catalyst used for preparation of chlorinated arylamines through catalytic hydrogenation
A technology for chlorinated aromatic amines and catalytic hydrogenation, which is applied in the preparation of organic compounds, catalysts for physical/chemical processes, and preparation of amino compounds. Hydrogen absorption and storage capacity, avoiding direct reduction, uniform distribution inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
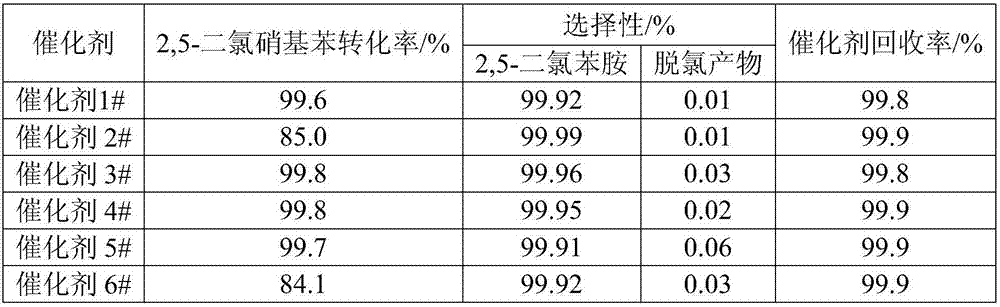
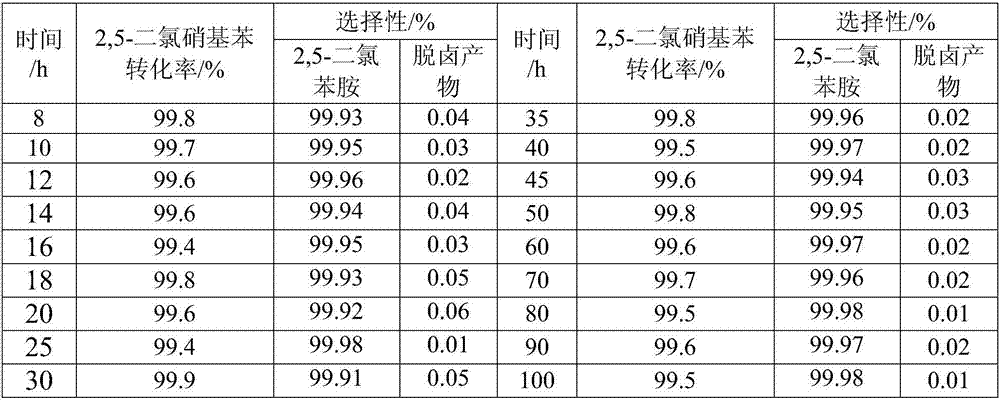
Examples
Embodiment 1
[0025] Coconut shell carbon with a particle size of 75 μm is used as the activated carbon carrier, and its specific surface area is 1500m 2 / g, the ash content is 1.5%. Weigh 50g of the above activated carbon, add 1000mL of 10wt% hydrochloric acid to it, stir at 60°C for 4h, filter to obtain a filter cake (activated carbon wet basis); add 400mL of 20wt% nitric acid to the activated carbon wet basis, heat and stir at 80°C for 4h, filter Obtain filter cake, wash with deionized water to neutrality, add 200mL deionized water to filter cake and be mixed with charcoal slurry.
[0026] Weigh 2.50g palladium chloride and dissolve it in 50mL of 20wt% dilute hydrochloric acid, add it to the carbon slurry, stir at 30°C for 2h, add 10wt% sodium hydroxide to adjust the pH=9.5, and continue to keep warm for 4h; Into H 2 6.5h, add 0.35g of ammonia water with a concentration of 10wt%, stir at 30°C for 6h, filter, wash the catalyst repeatedly with deionized water until the pH of the catalys...
Embodiment 2
[0028] Apricot shell charcoal with a particle size of 75 μm is used as the activated carbon carrier, and its specific surface area is 1200m 2 / g, the ash content is 2.0%. Weigh 50g of the above-mentioned activated carbon, add 800mL of 20wt% hydrochloric acid to it, stir at 60°C for 4h, and filter to obtain a filter cake (activated carbon wet basis); add 400mL of 30wt% nitric acid to the activated carbon wet basis, stir at 80°C for 4h, and obtain The filter cake was washed with deionized water until neutral, and 200 mL of deionized water was added to the filter cake to prepare a carbon slurry.
[0029] Weigh 1.83g of palladium nitrate and dissolve it in 50mL of 20wt% dilute hydrochloric acid, add it to the carbon slurry, stir at 30°C for 2h, add 10wt% sodium hydroxide to adjust the pH=9.5, and continue to keep warm for 4h; Access to H 2After 6.5 hours, continue to flow 0.11g of ammonia gas, stir at 30°C for 6 hours, filter, and wash the catalyst repeatedly with deionized wate...
Embodiment 3
[0031] Coconut shell carbon with a particle size of 75 μm is used as the activated carbon carrier, and its specific surface area is 1500m 2 / g, the ash content is 1.5%. Weigh 50g of the above-mentioned activated carbon, add 800mL of 15wt% hydrochloric acid to it, heat and stir at 60°C for 4h, filter to obtain a filter cake (activated carbon wet basis); add 800mL of 10wt% nitric acid to the activated carbon wet basis, heat and stir at 80°C for 4h, filter The obtained filter cake was washed with deionized water to neutrality, and 200 mL of deionized water was added to the filter cake to prepare carbon slurry.
[0032] Weigh 5.25g of chloroplatinic acid and dissolve it in 50mL of 20wt% dilute hydrochloric acid, add it to the carbon slurry, stir at 30°C for 2h, add 10wt% sodium hydroxide to adjust pH=10, and continue to keep warm for 4h; Into H 2 After 2h, add 0.86g of ammonium sulfate, stir at 30°C for 6h, filter, and wash the catalyst repeatedly with deionized water until the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com