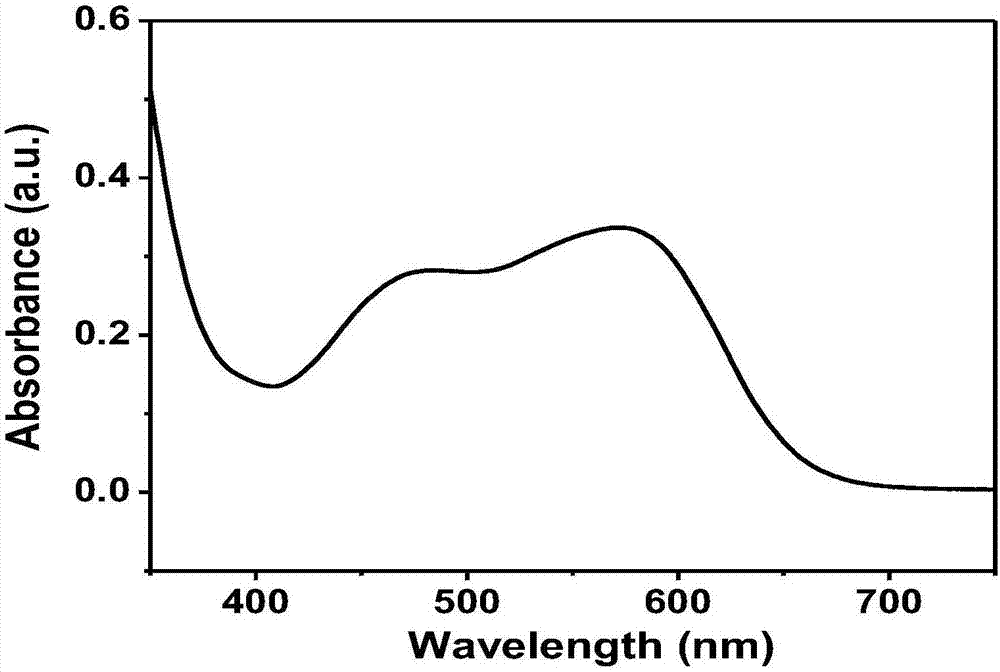
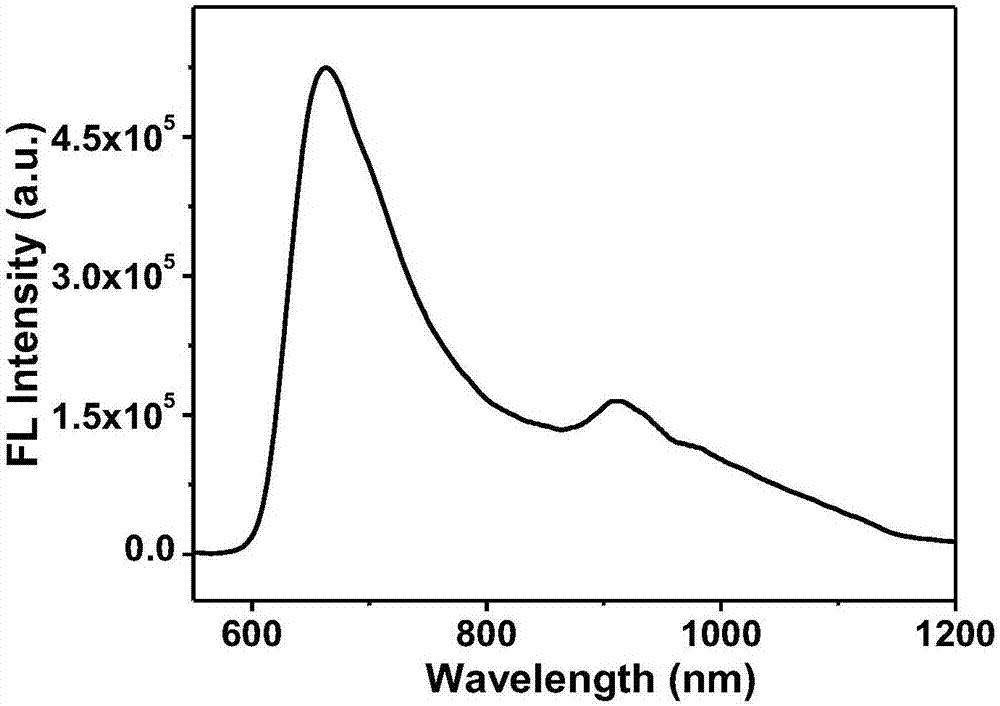
Preparation method of fluorescent dye and water-soluble near-infrared fluorescent probe
A technology of fluorescent probes and fluorescent dyes, applied in the field of chemical synthesis, can solve problems such as large damage to the human body and fine imaging of biological tissues, achieve good biocompatibility, high fluorescence quantum efficiency, and improve biocompatibility and specificity The effect of the recognition function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
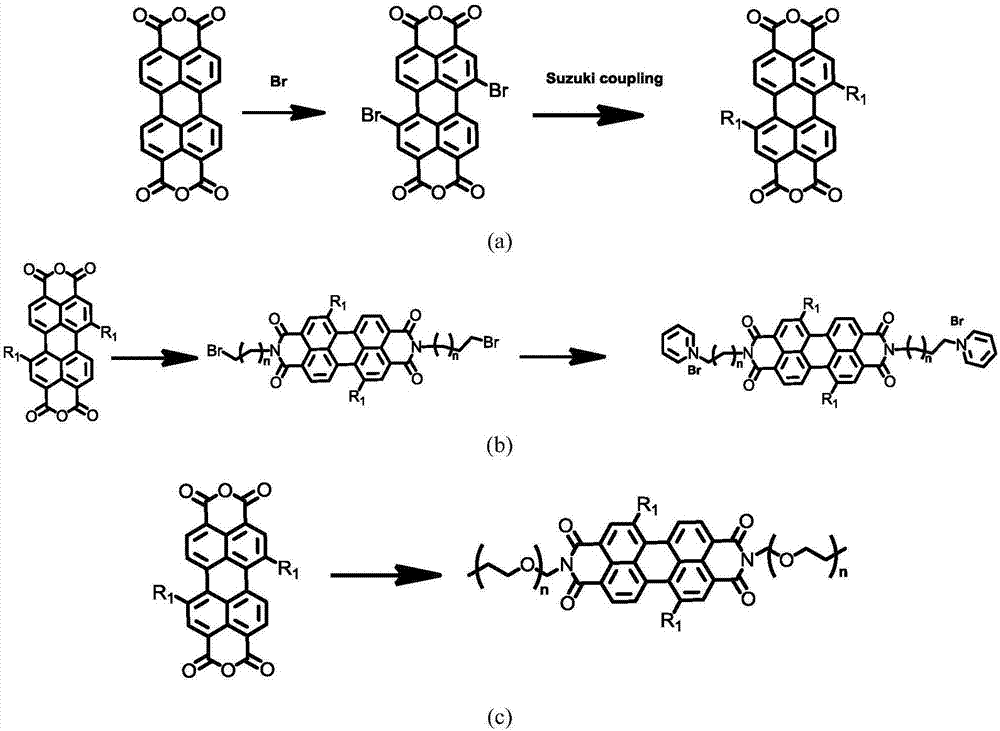
[0050] This embodiment is based on the method of step 1 and step 3 described in the present invention, such as figure 1N,N'-4-PEG(pentamer)phenyl-1,7-bis(4-(1,2,2-triphenyl)vinyl)benzene was prepared by replacing amine-terminated haloalkane with amine-terminated PEG as shown Base-3,4:9,10-tetracarboxylic perylenediamide.
[0051]
[0052] Perylene dianhydride (1.0 g) and 10 mL of concentrated sulfuric acid (98%) were added to a three-neck round bottom flask, and the mixture was stirred at 40° C. for 2 days. Bromine (1.3 mL) was added dropwise to the mixture over 30 minutes, followed by stirring at 100°C for 8 hours. After cooling to 40°C, the excess bromine was purged with argon with stirring. The mixture was then poured into ice water, the precipitate was separated by filtration, and the precipitate was washed with water several times until the filtrate was nearly neutral.
[0053] Preparation of cpd2. Trimethyltin-tetraphenylethylene (550mg), cpd1 (300mg) and Pd (PPh ...
Embodiment 2
[0056] In this example, based on the methods of step 1 and step 3 of the present invention, N, N'-2,4,6-tripolyvinyl alcohol phenyl-1,7-di (4-(1,2,2-Triphenyl)vinyl)phenyl-3,4:9,10-tetracarboxylic perylenediamide.
[0057]
[0058] Perylene dianhydride (10.0 g) and 20 mL of concentrated sulfuric acid (98%) were added to a three-neck round bottom flask, and the mixture was stirred at 60° C. for 2 days. Bromine (1.3 mL) was added dropwise to the mixture over 30 minutes, followed by stirring at 80°C for 8 hours. After cooling to 40°C, excess bromine was purged with nitrogen with stirring. The mixture was then poured into ice water, the precipitate was separated by filtration, and the precipitate was washed with water several times until the filtrate was nearly neutral.
[0059] Preparation of cpd2. Trimethyltin-tetraphenylethylene (2.15g), cpd1 (1.80g) and Pd(PPh 3 ) 4 (380 mg) was added to a double necked round bottom flask. The flask was evacuated under vacuum and flus...
Embodiment 3
[0062] In this example, based on the methods of step 1 and step 3 of the present invention, N, N'-2,4,6-pentapolyvinyl alcohol phenyl-1,7-di (4-(1,2,2-Triphenyl)vinyl)phenyl-3,4:9,10-tetracarboxylic perylenediamide.
[0063]
[0064] Preparation of 1,7-dibromo-3,4:9,10-tetracarboxylic perylene dianhydride (cpd1). Perylene dianhydride (5.0 g) and 15 mL of concentrated sulfuric acid (98%) were added to a three-neck round bottom flask, and the mixture was stirred at 60° C. for 3 days. Bromine (3.3 mL) was added dropwise to the mixture over 30 minutes, followed by stirring at 80°C for 5 hours. After cooling to 40°C, the excess bromine was purged with argon with stirring. The mixture was then poured into ice water, the precipitate was separated by filtration, and the precipitate was washed with water several times until the filtrate was nearly neutral.
[0065] Preparation of cpd2. Trimethyltin-tetraphenylethylene (4.34g), cpd1 (1.6g) and Pd(PPh 3 ) 4 (250 mg) was added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com