Red thermal induction delay fluorescent polymer and preparation and application thereof
A delayed fluorescence and polymer technology, applied in the manufacture of semiconductor/solid-state devices, luminescent materials, semiconductor devices, etc., can solve the problems of reduced radiation rate index rate, high non-radiative decay rate, and low photoluminescence efficiency of dyes, reaching The effect of suppressing efficiency roll-off, simple preparation method, and high external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The present invention also provides a preparation method of the red heat-induced delayed fluorescent polymer of the present invention, comprising:
[0085] Copolymerize the compound with the structure of formula (II), the compound with the structure of formula (III) and the compound with the structure of formula (IV) to obtain the polymer with the structure shown in formula (I);
[0086]
[0087] Wherein, Ar1 is 2,7-fluorene derivative or 2,7-carbazole derivative;
[0088] Ar2 is the aryl group of C6~C20;
[0089] R 1 , R 2 , R 3 , R 4 and R 5 independently selected from hydrogen, C1-C30 alkyl, C1-C30 alkoxy or C6-C50 substituted aryl;
[0090] x is 0
[0091] n is 1-200.
[0092] In the present invention, the compound having the structure of formula (II), the compound of formula (III) and the compound of formula (IV) are copolymerized to obtain the polymer of the structure shown in formula (I); wherein, R in the structure 1 , R 2 , R 3 , R 4 , R ...
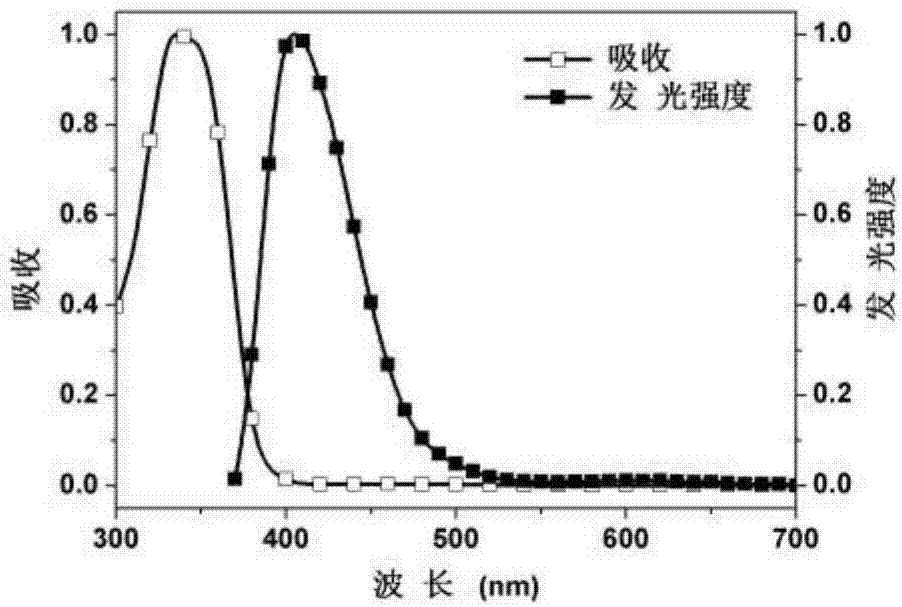
Embodiment 1
[0096] Embodiment 1: the synthesis of polymer PFSOTAQ0.5
[0097] The preparation process is shown in the following formula:
[0098]
[0099] The specific steps are:
[0100] 3,7-Dibromo-2,8-dioctyl-S,S-dioxo-dibenzothiophene (296.2mg, 0.495mmol), 9,9-dioctyl-2,7-dipinacol Borate fluorene (321.3mg, 0.5mmol), 2-(4-(bis(4-bromophenyl)amino)phenyl)-9,10-anthraquinone (3.0mg, 0.005mmol), palladium acetate ( 3mg) and tricyclohexylphosphine (6mg) were added to a 50mL Schlenk bottle, and the gas was changed three times, protected by argon, and deoxygenated toluene (8mL) was added, and heated at 82°C until the raw materials were completely dissolved; the deoxygenated tetraethyl A mixture of ammonium hydroxide (2mL) and water (2mL) was added to the reaction solution, and reacted at 80-85°C for 18h; phenylboronic acid (69mg, 0.6mmol) dissolved in 1mL of tetrahydrofuran was added to the reaction solution, and reacted for 6h, Add 1 mL of bromobenzene to the reaction solution and re...
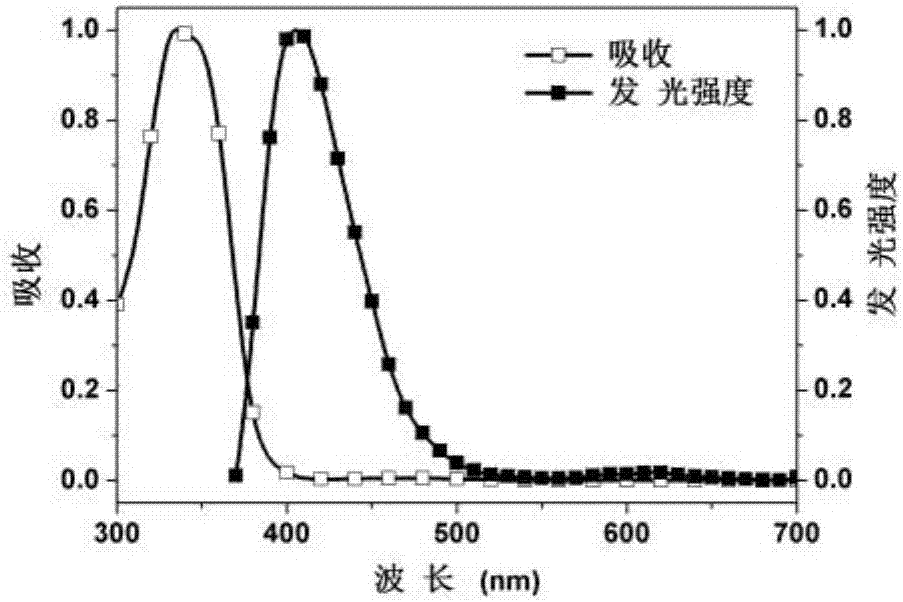
Embodiment 2
[0102] Example 2: Synthesis of Polymer PFSOTAQ1
[0103] The preparation process is shown in the following formula:
[0104]
[0105] The specific steps are:
[0106] 3,7-Dibromo-2,8-dioctyl-S,S-dioxo-dibenzothiophene (293.3mg, 0.49mmol), 9,9-dioctyl-2,7-dipinacol Borate fluorene (321.3mg, 0.5mmol), 2-(4-(bis(4-bromophenyl)amino)phenyl)-9,10-anthraquinone (6.1mg, 0.01mmol), palladium acetate ( 3mg) and tricyclohexylphosphine (6mg) were added to a 50mL Schlenk bottle, and the gas was changed three times, protected by argon, and deoxygenated toluene (8mL) was added, and heated at 82°C until the raw materials were completely dissolved; the deoxygenated tetraethyl A mixture of ammonium hydroxide (2mL) and water (2mL) was added to the reaction solution, and reacted at 80-85°C for 18h; phenylboronic acid (69mg, 0.6mmol) dissolved in 1mL of tetrahydrofuran was added to the reaction solution, and reacted for 6h, Add 1 mL of bromobenzene to the reaction solution and react for 6 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com