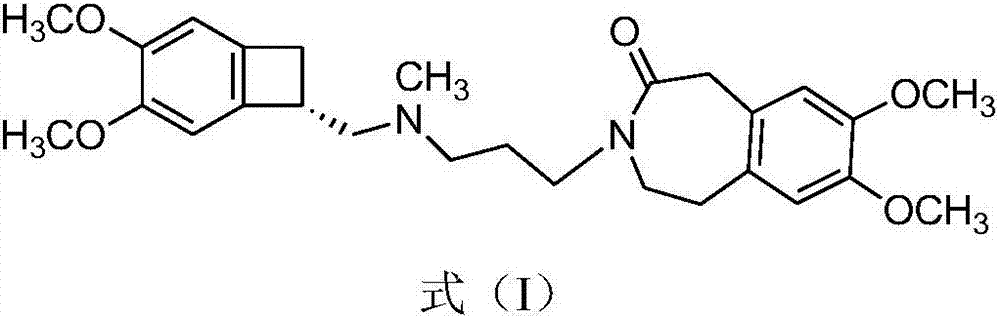
Synthetic method of ivabradine
A synthetic method and compound technology, applied in the field of medicine and chemical industry, to achieve good practical application value, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
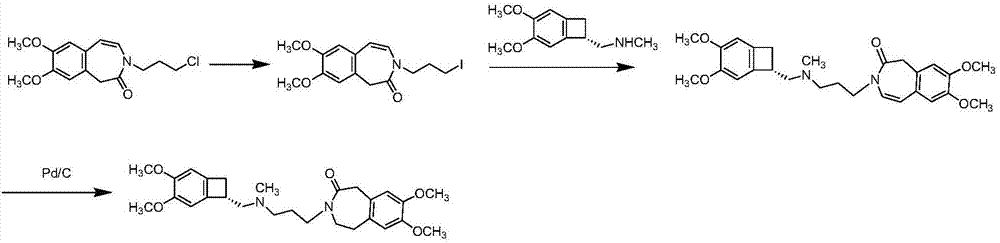
[0038] At room temperature, take 10g of compound (II) and 100ml of N,N-dimethylformamide and stir evenly, add 6.2g of potassium tert-butoxide to activate for 30min, then transfer it to a container containing 8.6g of bromochloropropane diluent, Control the temperature at 25±5°C, react for 30 minutes, transfer to 1L of ice water to quench the crystallization for 5 hours after the reaction is completed, filter, and recrystallize the crude product in acetone / water (volume ratio: 1:6) to obtain 9.8 g of white solid.
[0039] Add 8g of compound (III), 80ml of methanol, 14g of ammonium formate, and 1.6g of palladium carbon into the reaction flask, heat up to 60°C under normal pressure and react for 6h, filter, and concentrate the filtrate to dryness to obtain compound (IV).
[0040] Take 7g of compound (IV), 40ml of acetone, and 18g of sodium iodide, raise the temperature and reflux for 24 hours, cool and filter, concentrate to dryness under reduced pressure, add 50ml of dichlorometha...
Embodiment 2
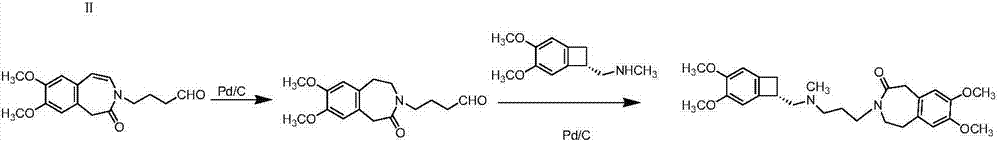
[0043] At room temperature, take 20g of compound (II) and 200ml of N,N-dimethylformamide and stir evenly, add 12.4g of potassium tert-butoxide to activate for 30min, then transfer it to a container containing 17.2g of bromochloropropane diluent, and control Temperature 25±5°C, react for 30min, transfer to 1L of ice water to quench the crystallization for 5h after the reaction is completed, filter, and recrystallize the crude product in acetone / water (volume ratio: 1:6) to obtain 20.1g of white solid.
[0044] Add 15g of compound (III), 200ml of methanol, 32g of ammonium formate, and 3.0g of palladium carbon into the reaction flask, heat up to 60°C under normal pressure and react for 6h, filter, and concentrate the filtrate to dryness to obtain compound (IV).
[0045] Get 12g of compound (IV), 80ml of acetone, 30g of sodium iodide, heat up and reflux for 24h, cool and filter, concentrate under reduced pressure to dryness, add 150ml of dichloromethane at room temperature and stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com