Heat-resistant alicyclic epoxy resin cured product and preparation method thereof
An epoxy resin curing and alicyclic technology, which is applied in the field of alicyclic epoxy resin, can solve the problems of unsatisfactory optical properties of cured products, inability to fully meet the requirements of high light transmittance, and difficulty in meeting the requirements of high heat resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
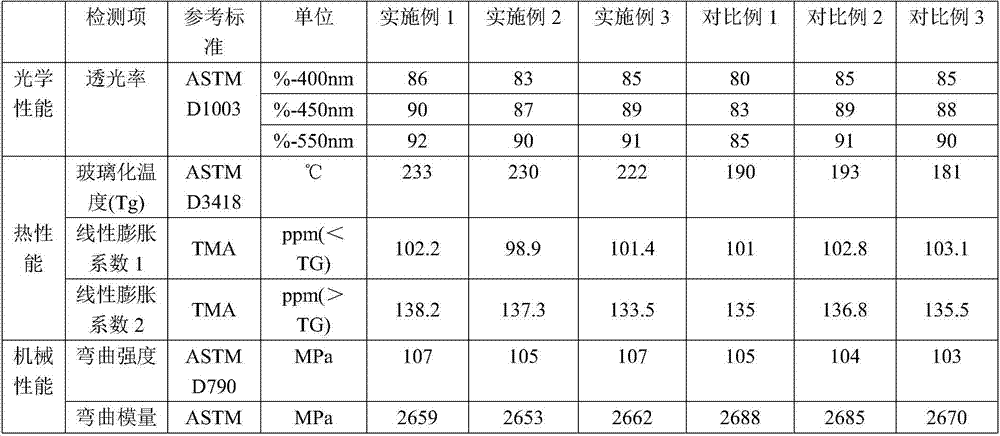
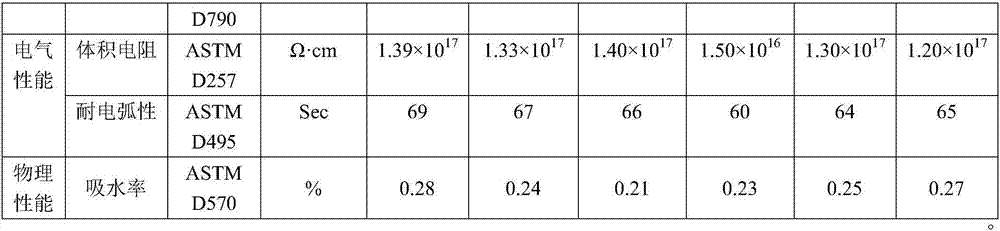
Examples
Embodiment 1
[0037] A kind of preparation method of 3,4-epoxycyclohexyl methyl 3,4-epoxycyclohexyl carboxylate, comprises the steps:
[0038] (1) Add 100g of 3-cyclohexene-1-carboxylic acid-3-cyclohexene-1-methyl ester, 200g of toluene, 106g of acetic anhydride and 32g of sodium acetate into a 1000ml three-necked flask, and cool to 10°C while stirring;
[0039] (2) Then start to drop 145g of 35% hydrogen peroxide, control the rate of addition to keep the temperature of the reaction system not exceeding 20°C-25°C, react for 4-8 hours, GC detects 3,4-epoxycyclohexylmethyl3,4 -Epoxy cyclohexyl carboxylate content is greater than 92% and then stops the reaction to obtain a reaction product;
[0040] (3) Slowly add 260 g of commercially available 30% NaOH solution to the reaction product to adjust the pH to 9-12, stir for 30 minutes and leave to separate the phases, and separate the upper organic phase;
[0041] (4) add 50g 10% sodium sulfite aqueous solution to the organic phase that step (3)...
Embodiment 2
[0046] A kind of preparation method of 3,4-epoxycyclohexyl methyl 3,4-epoxycyclohexyl carboxylate, comprises the steps:
[0047] (1) Add 100g of 3-cyclohexene-1-carboxylic acid-3-cyclohexene-1-methyl ester, 300g of toluene, 130g of acetic anhydride and 45g of sodium acetate into a 1000ml three-necked flask, and cool to 6°C while stirring;
[0048] (2) Then start to drop 176g of 35% hydrogen peroxide, control the rate of addition to keep the temperature of the reaction system not exceeding 20°C-25°C, react for 4-8 hours, GC detects 3,4-epoxycyclohexylmethyl3,4 -Epoxy cyclohexyl carboxylate content is greater than 92% and then stops the reaction to obtain a reaction product;
[0049] (3) Slowly add 320 g of commercially available 30% NaOH solution to the reaction product to adjust the pH to 9-12, stir for 30 minutes and leave to separate the phases, and separate the upper organic phase;
[0050] (4) add 60g 10% sodium sulfite aqueous solution to the organic phase that step (3) ob...
Embodiment 3
[0055] A kind of preparation method of 3,4-epoxycyclohexyl methyl 3,4-epoxycyclohexyl carboxylate, comprises the steps:
[0056] (1) Add 100g of 3-cyclohexene-1-carboxylic acid-3-cyclohexene-1-methyl ester, 250g of toluene, 120g of acetic anhydride and 40g of sodium acetate into a 1000ml three-necked flask, and cool to 15°C while stirring;
[0057] (2) Then start to drop 160g of 35% hydrogen peroxide, control the rate of addition to keep the temperature of the reaction system not exceeding 20°C-25°C, react for 4-8 hours, GC detects 3,4-epoxycyclohexylmethyl3,4 -Epoxy cyclohexyl carboxylate content is greater than 92% and then stops the reaction to obtain a reaction product;
[0058] (3) Slowly add 335 g of commercially available 30% NaOH solution to the reaction product to adjust the pH to 9-12, stir for 30 minutes and leave to separate phases, and separate the upper organic phase;
[0059] (4) add 55g 10% sodium sulfite aqueous solution to the organic phase that step (3) obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com