Method for detecting content of various biflavones in ginkgo leaf preparation
A technology of ginkgo biloba and biflavone, which is applied in the field of content determination of traditional Chinese medicine preparations, can solve the problems of long time consumption and low detection sensitivity, and achieve the effects of shortening detection time, controlling safety, and saving detection time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
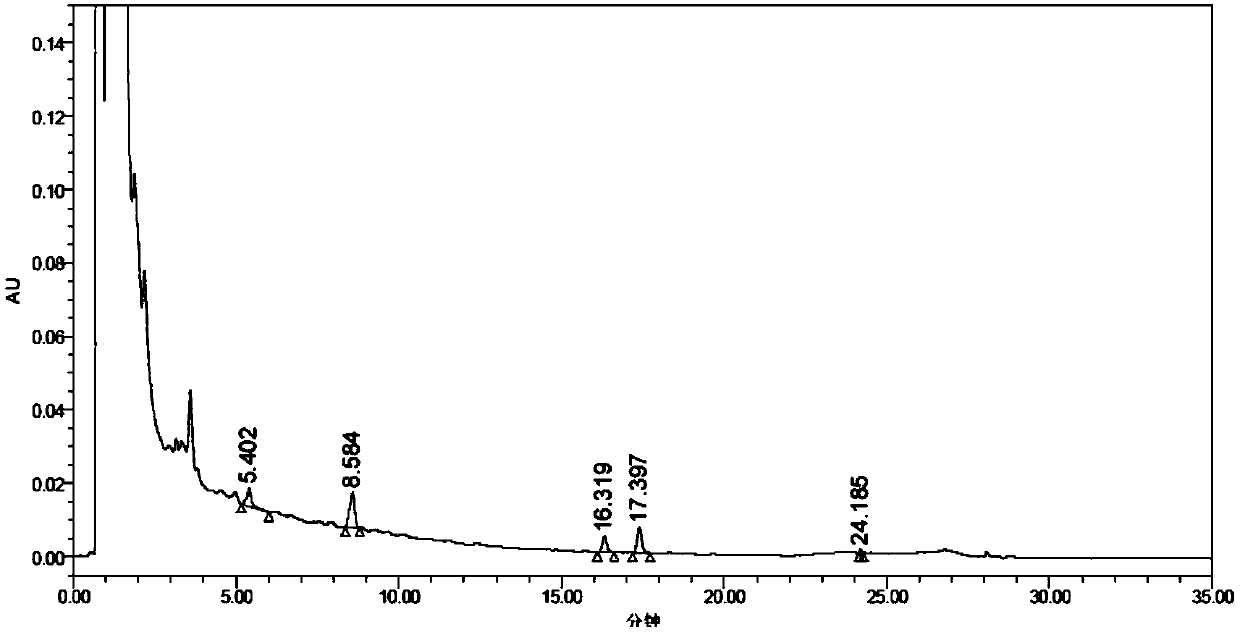
[0066] (1) High performance liquid chromatography conditions
[0067] Instrument: HPLC (Waters e2695);
[0068] Chromatographic system: Chromatographic column: Welch Bolimate C18 (100mm×3mm, 2.7μm);
[0069] Detection wavelength: 330nm; column temperature: 30°C; injection volume: 20μl; detector sensitivity: 0.04AUFS;
[0070] Mobile phase: flow rate 0.5ml / min.
[0071] The elution conditions are 0 minutes, 30% A; 0-20 minutes, 30%-45% A; 20-25 minutes, 45%-100% A; 25-30 minutes, 100%-30% A; 30-35 Minutes, 30% A.
[0072] (2) Preparation of standard solution:
[0073] (a) Standard product solution of Biflavones of P. chinensis: Accurately weigh 2mg of A. flavonoids of P. chinensis standard, dissolve them in 2ml of DMSO, shake well, take 1ml and dilute to 200ml with chromatographic methanol, and make 5.320μg / ml Abiflavones of P. chinensis Standard solution.
[0074] (b) Ginkgo biflavone standard solution: Accurately weigh 2mg of Ginkgo biflavone standard substance, dissolv...
Embodiment 2
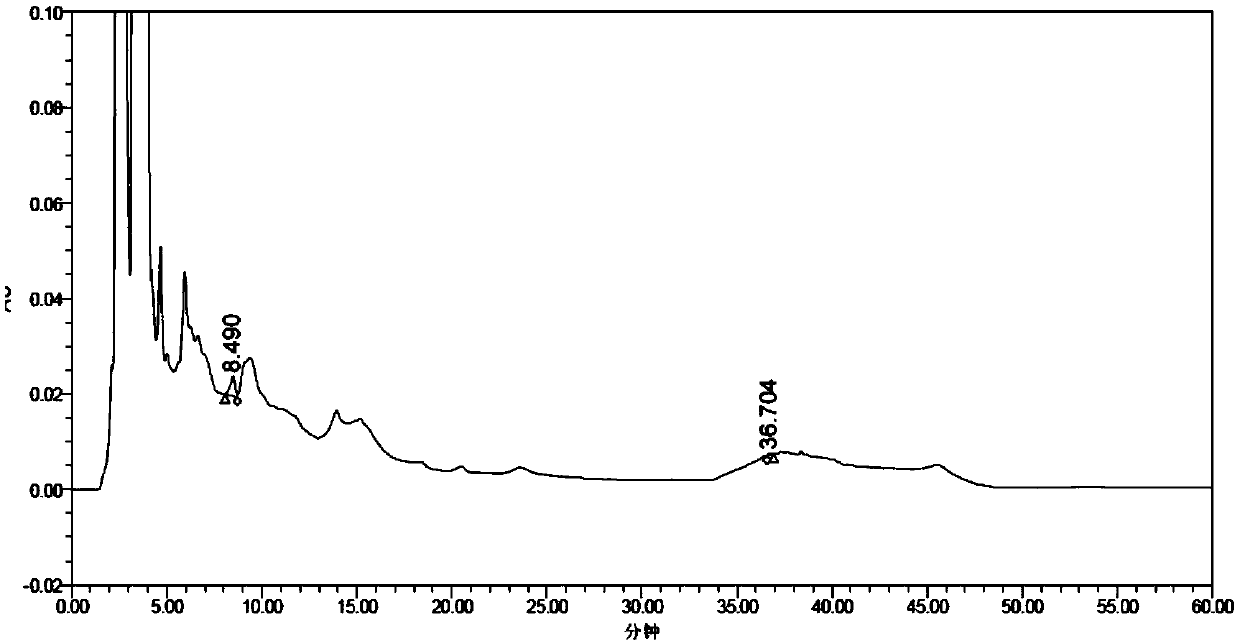
[0086] The difference from Example 1 is that the chromatographic column is: AgilentC18 (250mm×4.6mm, 5 μm); mobile phase: acetonitrile (A)-water (B) solution, gradient elution, the elution condition is 0 to 30 minutes, 47 %A; 30-33 minutes, 47%-80%A; 33-42 minutes, 80%A; 42-45 minutes, 80%-47%A; 45-55 minutes, 47%A; flow rate 1.0ml / min ; Detection wavelength: 270nm; Column temperature 40°C.
Embodiment 3
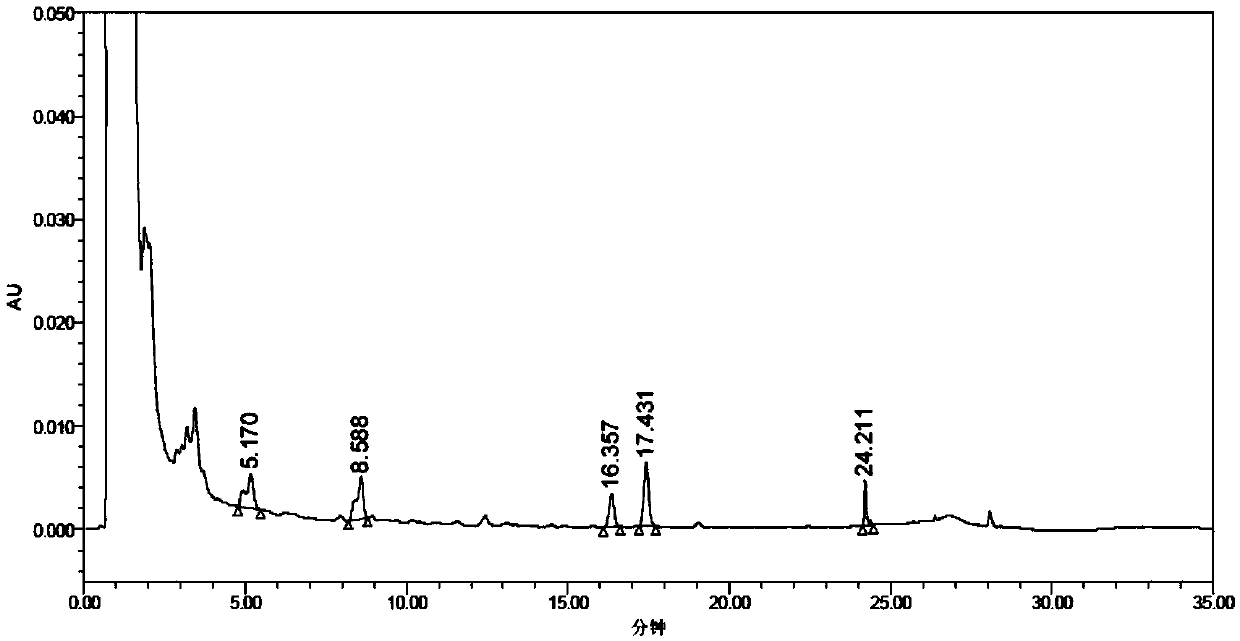
[0088] The same batch of Ginkgo Leaf Dropping Pills was used to verify the method of Example 1, and the sample solutions to be tested were respectively obtained for the determination of the biflavone content, and the precision was investigated. The results are shown in Table 3. The results showed that the RSD of assay met the requirement of RSD within 3%.
[0089] Table 3 The inventive method repeatability experiment content determination (unit: ppm)
[0090]
[0091] In the methodological verification of this method, the accuracy investigation (recovery rate experiment) meets the range requirements of 95% to 105%, and the RSD meets the requirements within 5%.
[0092] Experimental operation is the same as embodiment 1, and the results are shown in Table 4.
[0093] Table 4 The inventive method accuracy measurement
[0094]
[0095]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com