Pegylation benzoindoles heptamethine cyanine dye as well as preparation method and application thereof
A technology of benzindole heptamethine and pegylation, which is applied in the field of pegylated benzindole heptamethine dye and its preparation, can solve the problems of biomedical limitations and inability to directly dissolve, and achieve improvement Effects of biocompatibility, reduction of separation and purification costs, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) End-group iodopolyethylene glycol monomethyl ether PEG 16 -I preparation
[0030] Add 22.0g (0.04mol) of dried polyethylene glycol monomethyl ether (Mn=550) into a 500mL round-bottom flask, and add 150mL of anhydrous tetrahydrofuran solvent to dissolve it in N 2 Under the protection of inert gas, add 8.08g (0.08mol) triethylamine basic catalyst, control the temperature at 0~5℃, slowly add 20g (0.08mol) methanesulfonyl chloride dissolved in 30mL tetrahydrofuran solution, add dropwise and stir at room temperature for reaction After 10 hours, the insoluble matter was removed by filtration and the organic solvent was removed under reduced pressure to obtain a white solid. Add 40mL of tetrahydrofuran to dissolve the solid, slowly add the solution dropwise to 400mL of cold ether, resulting in a white precipitate. After the ether solution is poured out, the white solid sticking to the wall is dissolved in tetrahydrofuran, and the cold ether is precipitated. Repeat 3 to 4 times...
Embodiment 2
[0039] (1) End-group brominated polyethylene glycol monomethyl ether PEG 16 -Br preparation
[0040] Add 30.0g (0.04mol) of dried polyethylene glycol monomethyl ether (Mn=750) into a 500mL round bottom flask, and add 200mL of anhydrous tetrahydrofuran solvent to dissolve it, 2 Add 8.08g (0.08mol) triethylamine basic catalyst under the protection of inert gas, and slowly add 9.20g (0.08mol) methanesulfonyl chloride dissolved in 30mL dry tetrahydrofuran after the temperature is controlled at 0~5℃. Then, the reaction was stirred at room temperature for 10 hours, the insoluble matter was removed by filtration and the organic solvent was removed under reduced pressure to obtain a white solid. Add 40mL of tetrahydrofuran to dissolve the solid, slowly drop the solution into 400mL of cold ether, a white precipitate is generated, after the ether solution is poured out, the white solid sticking to the wall continues to be dissolved with tetrahydrofuran, and the cold ether is precipitated. R...
Embodiment 3
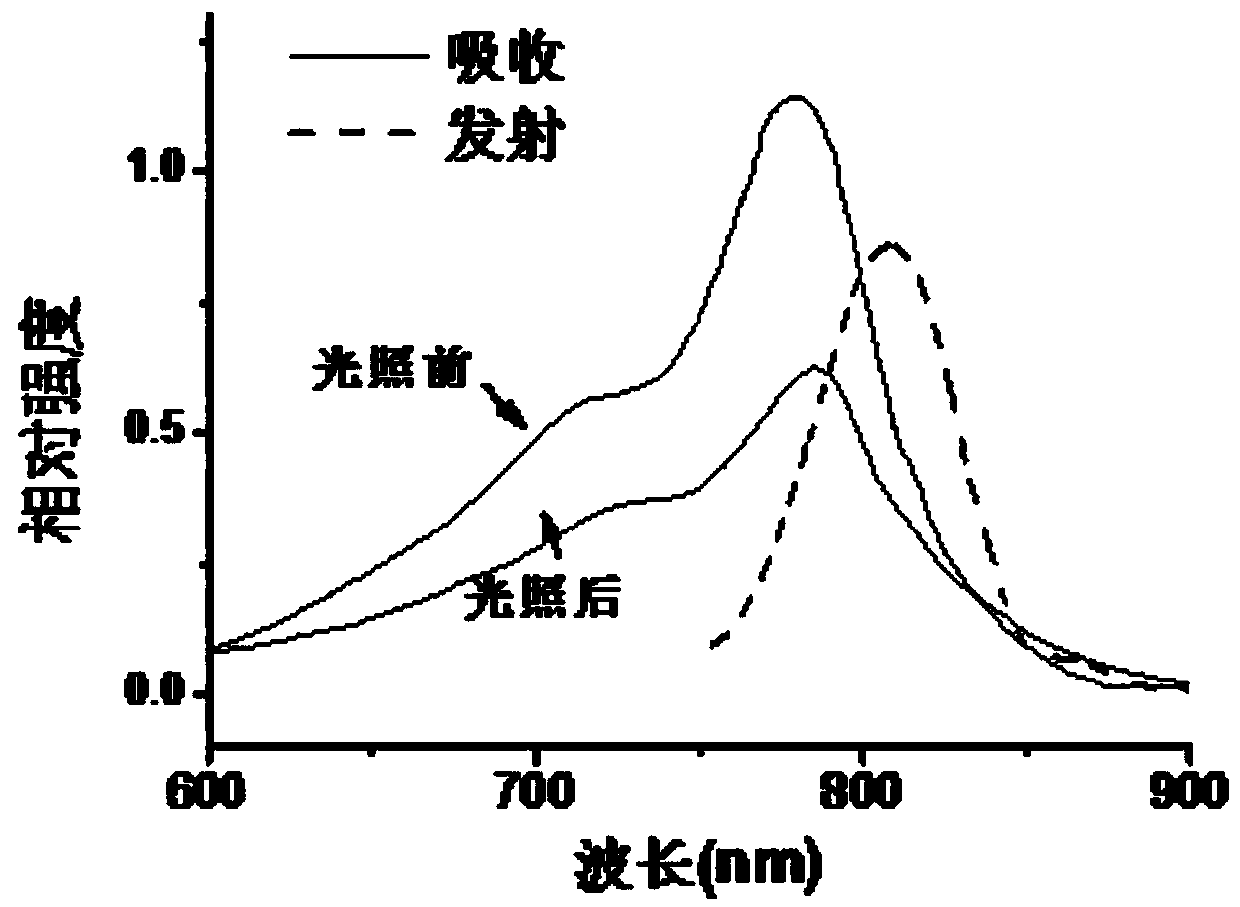
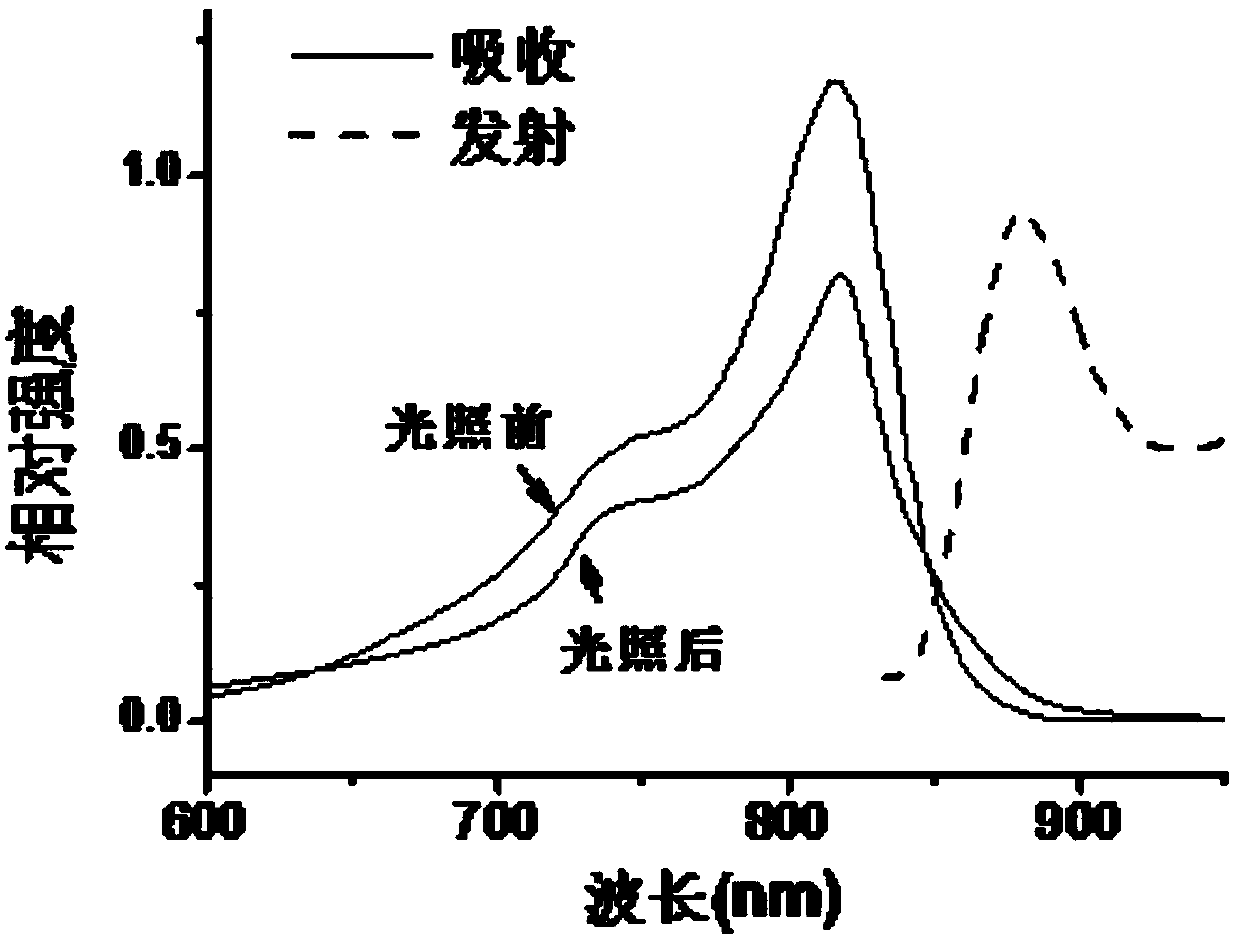
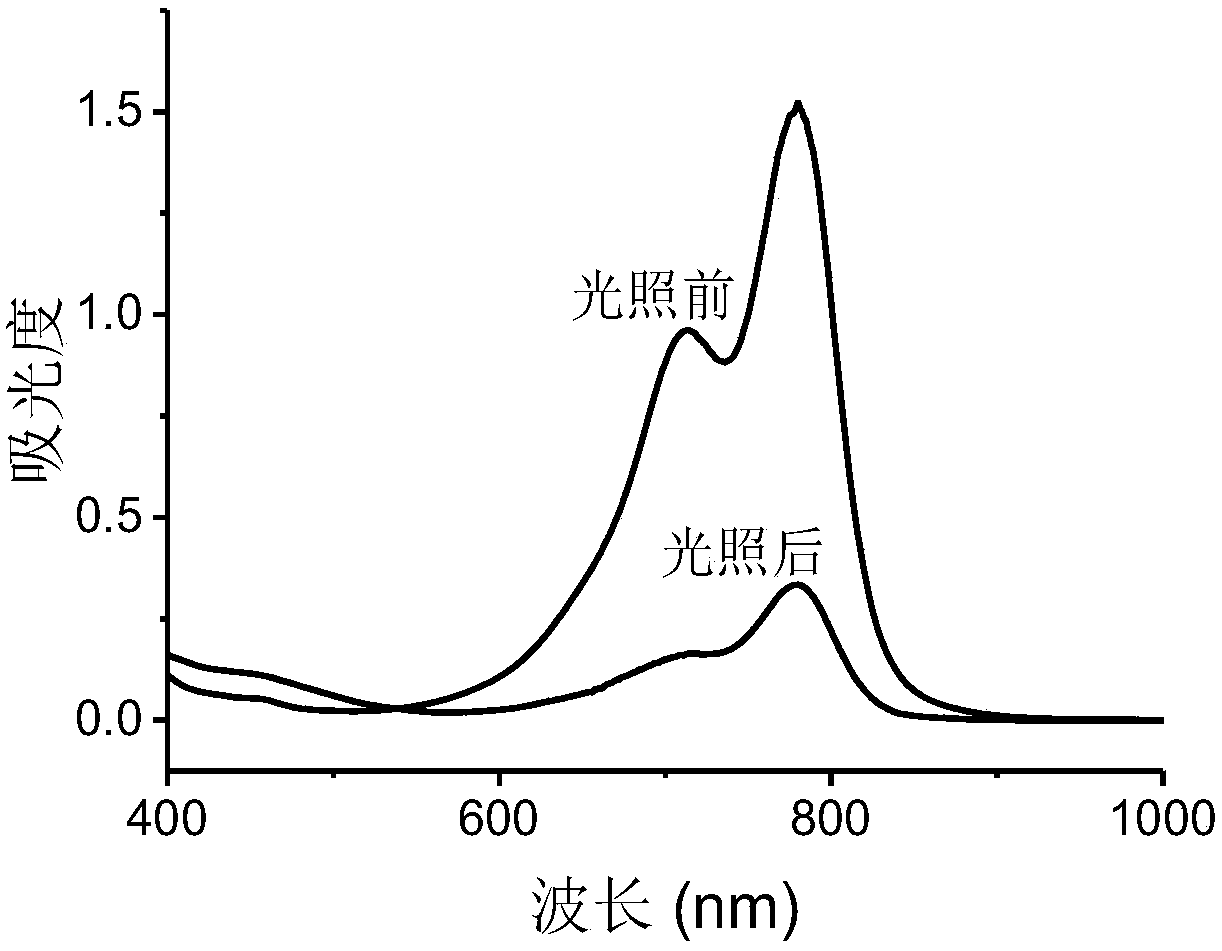
[0049] The visible-near infrared absorption spectrum and fluorescence emission spectrum of the IR-PEG(a) and IR-PEG(b) aqueous solutions of Example 1 and Example 2 were measured. In order to evaluate the light stability, the laser intensity was 0.6W cm -2 The dye solution was irradiated with the 808nm laser for 5 minutes, and the visible-near infrared absorption spectrum after the laser irradiation was measured. In order to compare with the light stability of ICG, the visible-near infrared absorption spectra of the aqueous ICG solution before and after laser irradiation were measured at the same time.
[0050] Prepare IR-PEG(a), IR-PEG(b) and ICG into 50μM aqueous solution, using 0.8W cm -2 A laser with a wavelength of 808nm was irradiated, and the instant temperature of the dye solution was monitored with a thermal imager. The blank solution was used for comparison.
[0051] figure 1 with figure 2 These are the ultraviolet absorption spectra and fluorescence emission spectra of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com