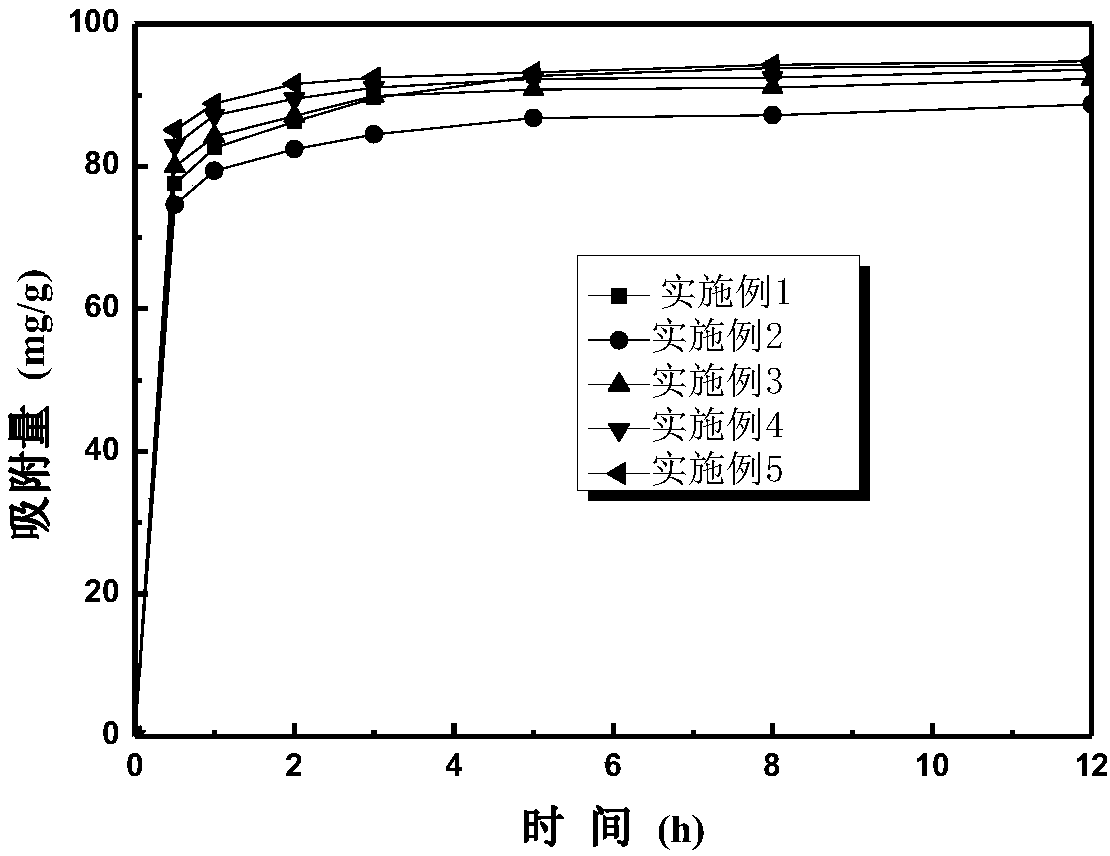
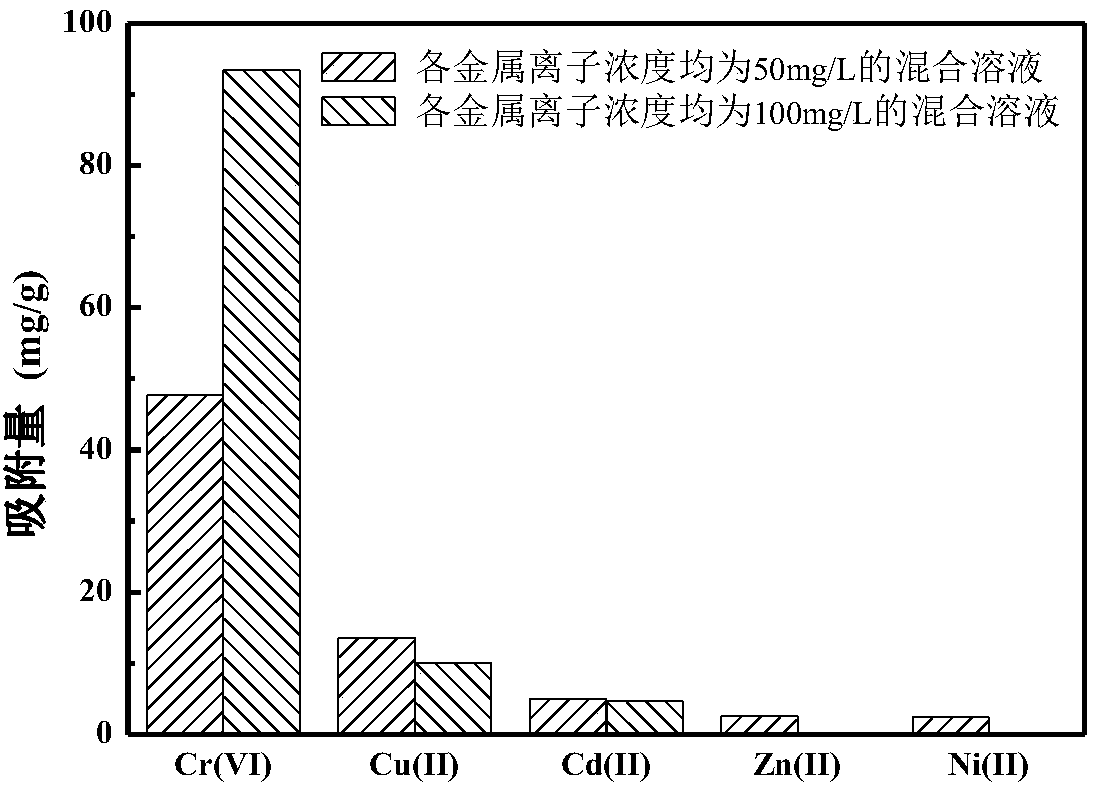
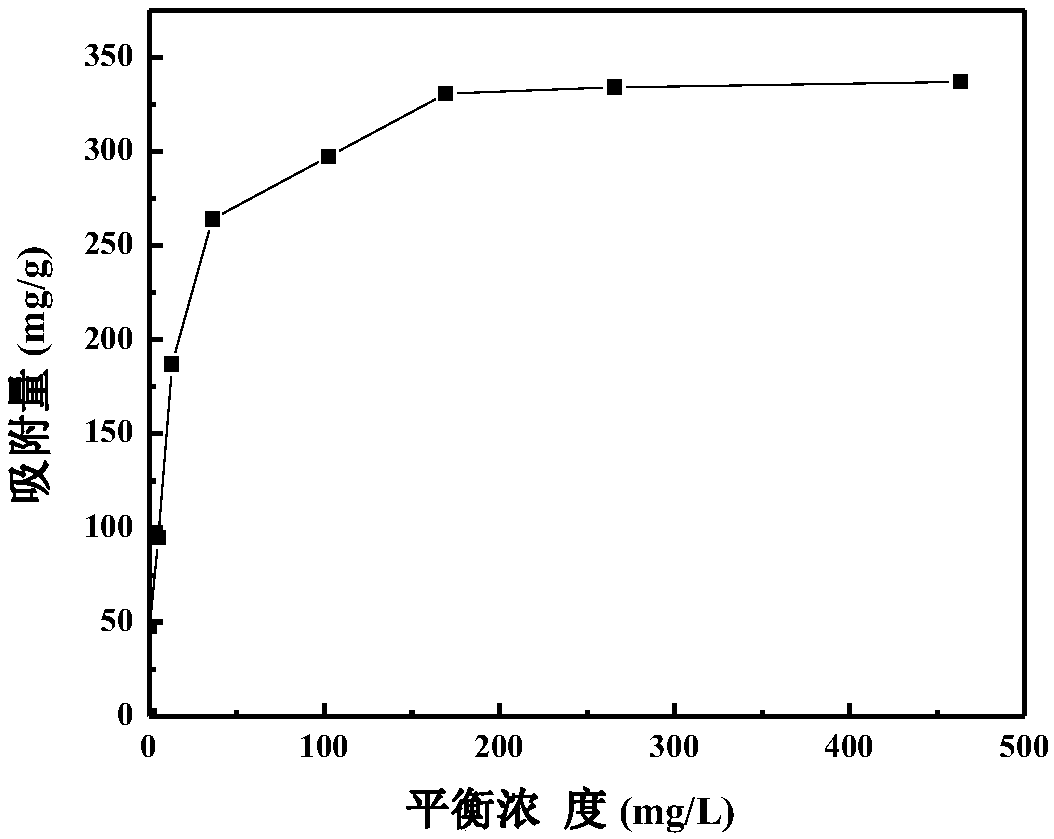
Preparation method of magnetic chitosan adsorbent
A technology of adsorbent and chitosan, which is applied in the field of preparation and application of magnetic chitosan composite materials, achieves the effect of simple preparation method, low equipment requirements and saving separation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Dissolve 2.7g of ferric chloride hexahydrate in 80mL of ethylene glycol (analytical grade), stir until the solution is clear and transparent, add 7.2g of anhydrous sodium acetate, and continue stirring until it dissolves; React for 15 hours; after the reaction is completed, remove the supernatant, wash with absolute ethanol and deionized water for 2-3 times after magnetic nuclear magnetic separation, and vacuum dry at 60° C. for 12 hours.
[0035] Add 0.2g of magnetic nuclei prepared by the above steps into a mixture of 60mL of absolute ethanol and 80mL of deionized water, and sonicate for 10min until the magnetic nuclei are evenly dispersed; add 1140μL of ammonia water and 0.28g of CTAB into the above liquid, and stir for 30min; 400 μL of hexyl orthosilicate (analytical grade), stirred for 5 hours; the resulting black solid was separated by magnetic force, washed and dried in vacuum at 60°C for 12 hours.
[0036] At room temperature, disperse 0.2g of silica-coated magn...
Embodiment 2
[0039] Dissolve 2.7g of ferric chloride hexahydrate in 80mL of ethylene glycol (analytical grade), stir until the solution is clear and transparent, add 7.2g of anhydrous sodium acetate, and continue stirring until it dissolves; React for 16 hours; after the reaction is completed, remove the supernatant, wash with absolute ethanol and deionized water for 2-3 times after magnetic nuclear magnetic separation, and vacuum dry at 60° C. for 12 hours.
[0040] Add 0.2g of magnetic nuclei to a mixture of 60mL of absolute ethanol and 80mL of deionized water, sonicate for 10min until the magnetic nuclei are evenly dispersed; add 1140μL of ammonia water and 0.28g of cetyltrimethylammonium bromide to the above liquid, and stir for 30min ; Add 400 μL hexyl orthosilicate solution (analytical grade) dropwise, stir for 5.5 hours and then magnetically separate the resulting black solid, wash with absolute ethanol and deionized water for 2-3 times, and then vacuum-dry at 60°C for 12 hours.
[...
Embodiment 3
[0044] Dissolve 2.7g of ferric chloride hexahydrate in 80mL of ethylene glycol (analytical grade), stir until the solution is clear and transparent, add 7.2g of anhydrous sodium acetate, and continue stirring until it dissolves; React for 17 hours; after the reaction is completed, remove the supernatant, wash with absolute ethanol and deionized water for 2-3 times after magnetic nuclear magnetic separation, and vacuum dry at 60° C. for 12 hours.
[0045] Add 0.2g of magnetic nuclei to a mixture of 60mL of absolute ethanol and 80mL of deionized water, sonicate for 10min until the magnetic nuclei are evenly dispersed; add 1140μL of ammonia water and 0.28g of cetyltrimethylammonium bromide to the above liquid, and stir for 30min ; Add 400 μL of hexyl orthosilicate solution (analytical grade) dropwise, stir for 6 hours, and magnetically separate the obtained black solid, wash with absolute ethanol and deionized water for 2-3 times, and then vacuum-dry at 60°C for 12 hours.
[0046...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com