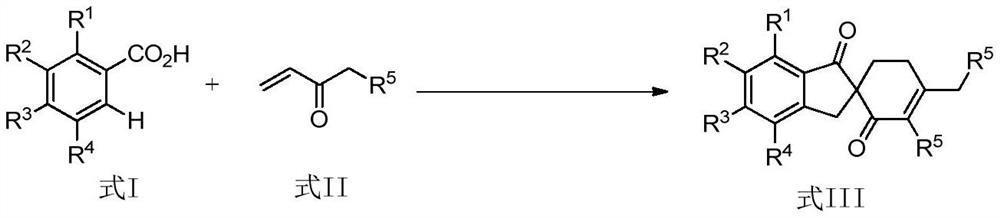A kind of synthetic method containing 1-indanone skeleton spiro compound
A synthesis method and compound technology, applied in the field of synthesis of spirocyclic compounds containing 1-indanone skeleton, to achieve the effects of easy-to-obtain raw materials, simple reaction operation, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
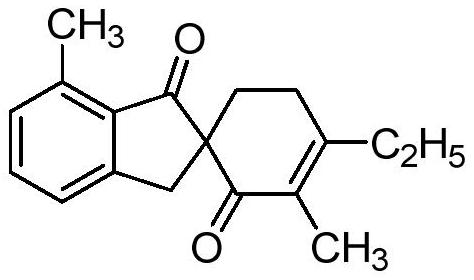
[0018] Add 13.6mg (0.1mmol) o-toluic acid, 25μL (0.25mmol) 1-penten-3-one, 3.1mg (0.0050mmol) p-cymene dichloride ruthenium dimerization to a 10mL pressure-resistant reaction tube solid, 13.0mg (0.0750mmol) anhydrous manganese acetate, 0.6mL acetonitrile, stirred and reacted at 150°C for 20 hours under the protection of argon, cooled to room temperature after the reaction, and filtered by column chromatography silica gel column to remove the catalyst and some insoluble salts , separated by thin-layer chromatography to obtain a spirocyclic compound containing a 1-indanone skeleton with the following structural formula:
[0019]
[0020] Its yield is 62%, and the structural characterization data are as follows:
[0021] 1 H NMR (400MHz, CDCl 3 ): δ[ppm]=7.41(t, J=7.5Hz, 1H), 7.24(d, J=8.4Hz, 1H), 7.08(d, J=7.4Hz, 1H), 3.65(d, J=17.0 Hz,1H),2.88–2.76(m,2H),2.58(s,3H),2.42–2.25(m,4H),2.11–2.02(m,1H),1.79(s,3H),1.13(t, J=7.6Hz, 3H).
[0022] 13 C NMR (100MHz, CDCl 3 ): δ[...
Embodiment 2
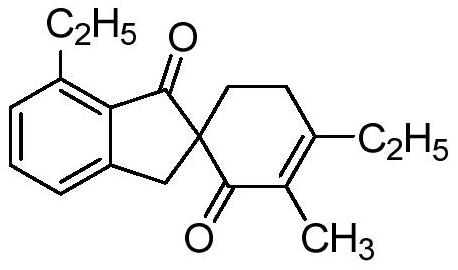
[0025] The o-toluic acid used in embodiment 1 is replaced with o-ethylbenzoic acid of equimolar amount, and other steps are identical with embodiment 1, obtain the following structural formula containing 1-indanone skeleton spiro compound:
[0026]
[0027] Its yield is 58%, and the structural characterization data are as follows:
[0028] 1 H NMR (400MHz, CDCl 3 ): δ[ppm]=7.45(t, J=7.5Hz, 1H), 7.25(d, J=6.7Hz, 1H), 7.13(d, J=7.5Hz, 1H), 3.66(d, J=16.9 Hz,1H),3.03(qd,J=7.3,2.3Hz,2H),2.83(d,J=17.0Hz,2H),2.41–2.25(m,4H),2.12–2.01(m,1H),1.80 (s, 3H), 1.19 (t, J = 7.5Hz, 3H), 1.13 (t, J = 7.6Hz, 3H).
[0029] 13 C NMR (100MHz, CDCl 3 ): δ[ppm]=204.9, 197.1, 161.3, 153.9, 146.0, 134.4, 132.0, 129.3, 127.6, 123.7, 59.6, 37.6, 31.5, 28.4, 26.7, 24.8, 14.7, 11.7, 10.8.
[0030] HRMS(ESI)m / z:C 19 h 22 o 2 ,[M+Na] + , The theoretical value is 305.1517; the measured value is 305.1506.
Embodiment 3
[0032] The o-toluic acid used in Example 1 is replaced with an equimolar amount of 2,3-dimethylbenzoic acid, and the other steps are the same as in Example 1 to obtain a 1-indanone skeleton-containing spiro compound with the following structural formula :
[0033]
[0034] Its yield is 61%, and the structural characterization data are as follows:
[0035] 1 H NMR (400MHz, CDCl 3 ): δ[ppm]=7.32(d, J=7.7Hz, 1H), 7.15(d, J=7.7Hz, 1H), 3.57(d, J=16.8Hz, 1H), 2.85–2.77(m, 2H ),2.55(s,3H),2.40–2.31(m,4H),2.28(s,3H),2.09–2.01(m,1H),1.80(s,3H),1.13(t,J=7.6Hz, 3H).
[0036] 13 C NMR (100MHz, CDCl 3 ): δ[ppm]=205.6, 197.2, 161.1, 151.5, 137.8, 136.4, 136.0, 132.5, 129.2, 123.1, 59.9, 36.9, 31.4, 28.4, 26.7, 18.9, 13.6, 11.6, 10.7.
[0037] HRMS(ESI)m / z:C 19 h 22 o 2 ,[M+Na] + , The theoretical value is 305.1517; the measured value is 305.1519.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com