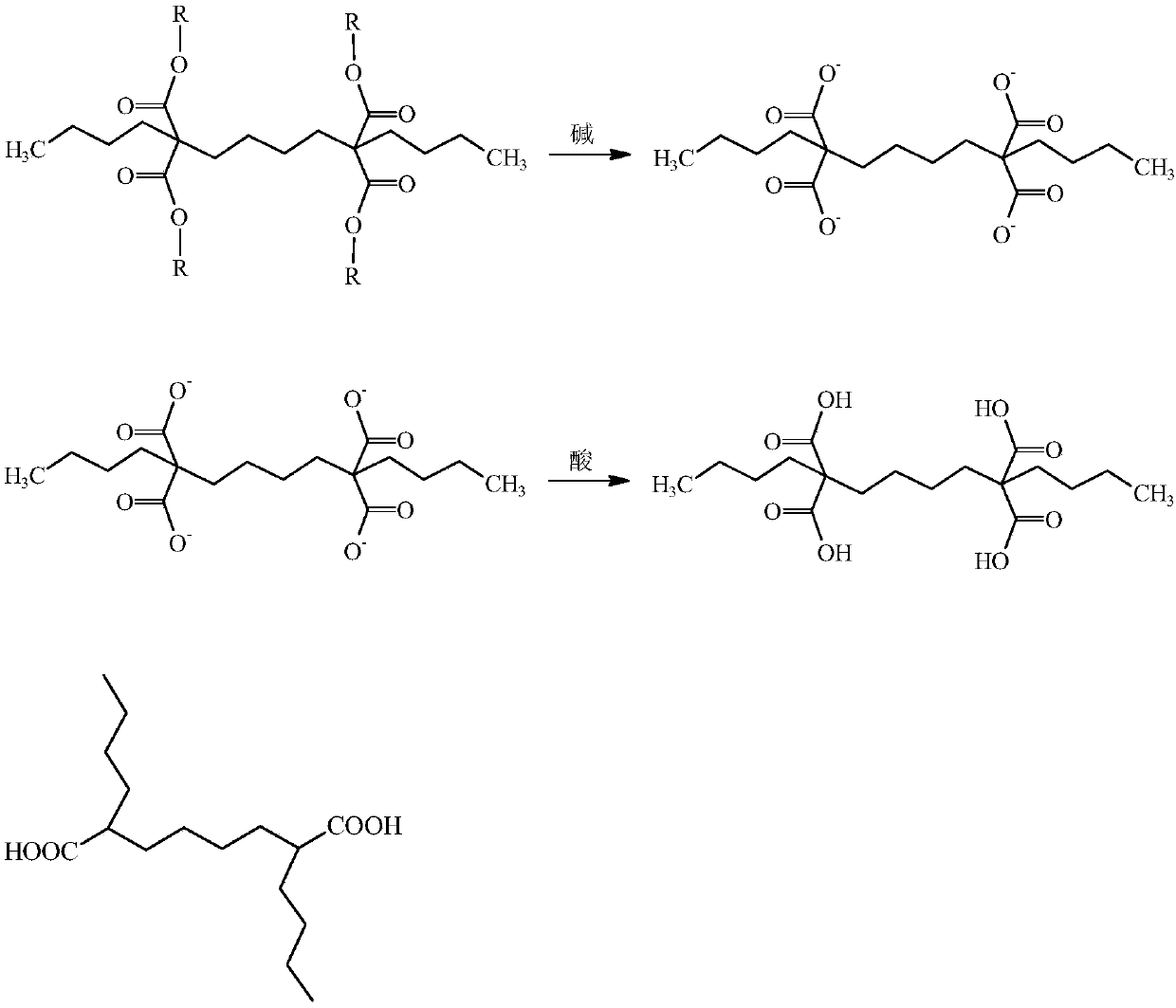
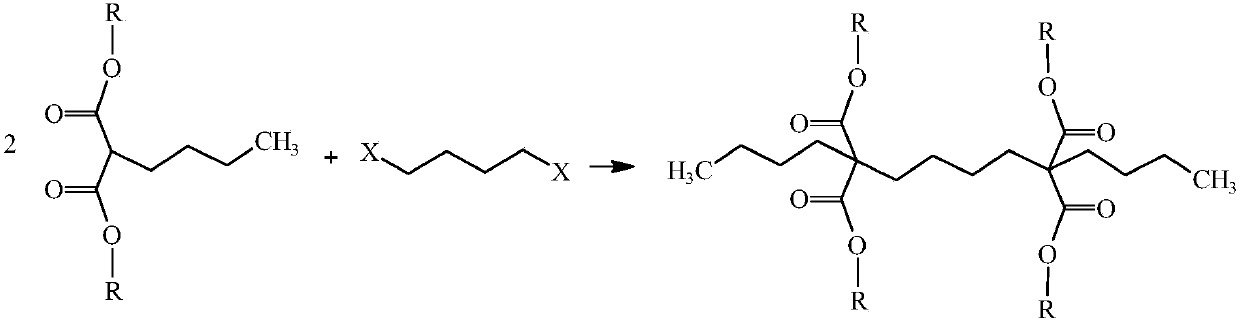
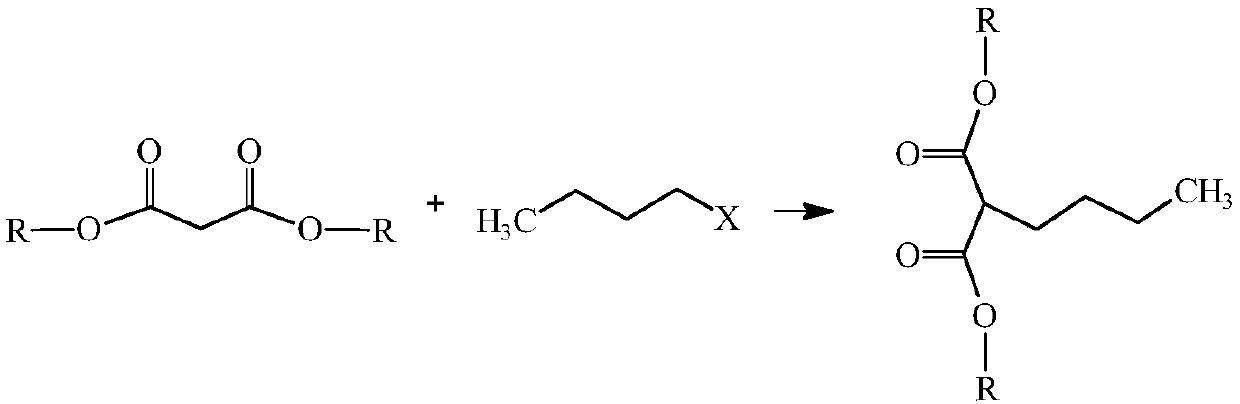
Preparation method of capacitor-grade 2,7-dibutyl octanedioic acid
A technology of dialkoxycarbonyl suberic acid diester and n-butyl malonate diester, which is applied in the synthesis of 2,7-dibutyl suberic acid by phase transfer catalysis, capacitor grade 2,7-di The production field of butyl suberic acid can solve the problems of harsh reaction conditions, troublesome post-processing, complicated process, etc., and achieve the effect of short reaction time, fast reaction speed and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step (1): 160.2Kg (1Kmol) diethyl malonate, 101.8Kg (1.1Kmol) chlorobutane, 165.9Kg (1.2Kmol) potassium carbonate and 16.1Kg (0.05Kmol) tetrabutylammonium bromide Add it into the reaction kettle, heat to reflux under stirring, react for 2h, after the reaction is completed, cool and filter, and the filtrate is distilled to remove unreacted diethyl malonate and chlorobutane to obtain n-butyl propane The mixed solution of diethyl diethyl malonate and phase transfer agent was used for subsequent use, and the yield of diethyl n-butylmalonate was 78.6%.
[0034]Step (2): 170Kg (0.786Kmol) n-butyl diethyl malonate obtained above, 16.1Kg (0.05Kmol) tetrabutylammonium bromide, 67.9Kg (0.31Kmol) 1,4-dibromobutyl Alkanes, 150Kg (0.9Kmol) of potassium carbonate were added to the reactor, heated to reflux under stirring, reacted for 4h, after the reaction was completed, cooled and filtered, the filtrate was rectified to obtain 2,7-dibutyl-2,7-di Diethyl ethoxycarbonyl suberate, the...
Embodiment 2
[0038] Step (1): 132.2Kg (1Kmol) dimethyl malonate, 103.7Kg (1.12Kmol) chlorobutane, 358.4Kg (1.1Kmol) cesium carbonate and 14.6Kg (0.04Kmol) cetyl trimethyl Ammonium bromide was added to the reaction kettle, heated to reflux under stirring, and reacted for 2.5h. After the reaction was completed, it was cooled and filtered, and the filtrate was distilled to remove unreacted dimethyl malonate and chlorobutane to obtain The mixed solution of dimethyl n-butylmalonate and phase transfer agent is ready for use, and the yield of dimethyl n-butylmalonate is 80.5%.
[0039] Step (2): 151.5Kg (0.805Kmol) dimethyl n-butylmalonate, 14.6Kg (0.04Kmol) hexadecyltrimethylammonium bromide, 78.2Kg (0.36Kmol) 1 obtained above, Add 4-dibromobutane and 275.4Kg (0.85Kmol) cesium carbonate into the reactor, heat to reflux under stirring, react for 3.5h, after the reaction is completed, cool and filter, and the filtrate is rectified to obtain 2,7-dibutyl Dimethyl-2,7-dimethoxycarbonyl suberate with...
Embodiment 3
[0043] Step (1): 188.2Kg (1Kmol) dipropyl malonate, 111.1Kg (1.2Kmol) chlorobutane, 193.5Kg (1.4Kmol) potassium carbonate and 32Kg (0.1Kmol) hexadecyl trimethyl Ammonium chloride was added to the reaction kettle, heated to reflux under stirring, and reacted for 1 hour. After the reaction was completed, it was cooled and filtered, and the filtrate was distilled to remove unreacted dipropyl malonate and chlorobutane to obtain n-butyl The mixed solution of dipropyl butyl malonate and phase transfer agent is standby, and the yield of dipropyl n-butyl malonate is 72.5%.
[0044] Step (2): 177.1Kg (0.725Kmol) n-butyl malonate dipropyl ester obtained above, 32Kg (0.1Kmol) cetyltrimethylammonium chloride, 78.2Kg (0.36Kmol) 1,4 - Dibromobutane and 120.2Kg (0.87Kmol) potassium carbonate were added to the reactor, heated to reflux under stirring, and reacted for 2h. After the reaction was completed, cooled and filtered, the filtrate was rectified to obtain 2,7-dibutyl- Dipropyl 2,7-dipr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com