Cyclic trimetric polybutylene terephthalate preparation method
A technology for polybutylene terephthalate and terephthalate, which is applied in the field of compound preparation, can solve the problems of long synthesis process, high cost, complicated operation and the like, and achieves short synthesis route, simple operation and conditions easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
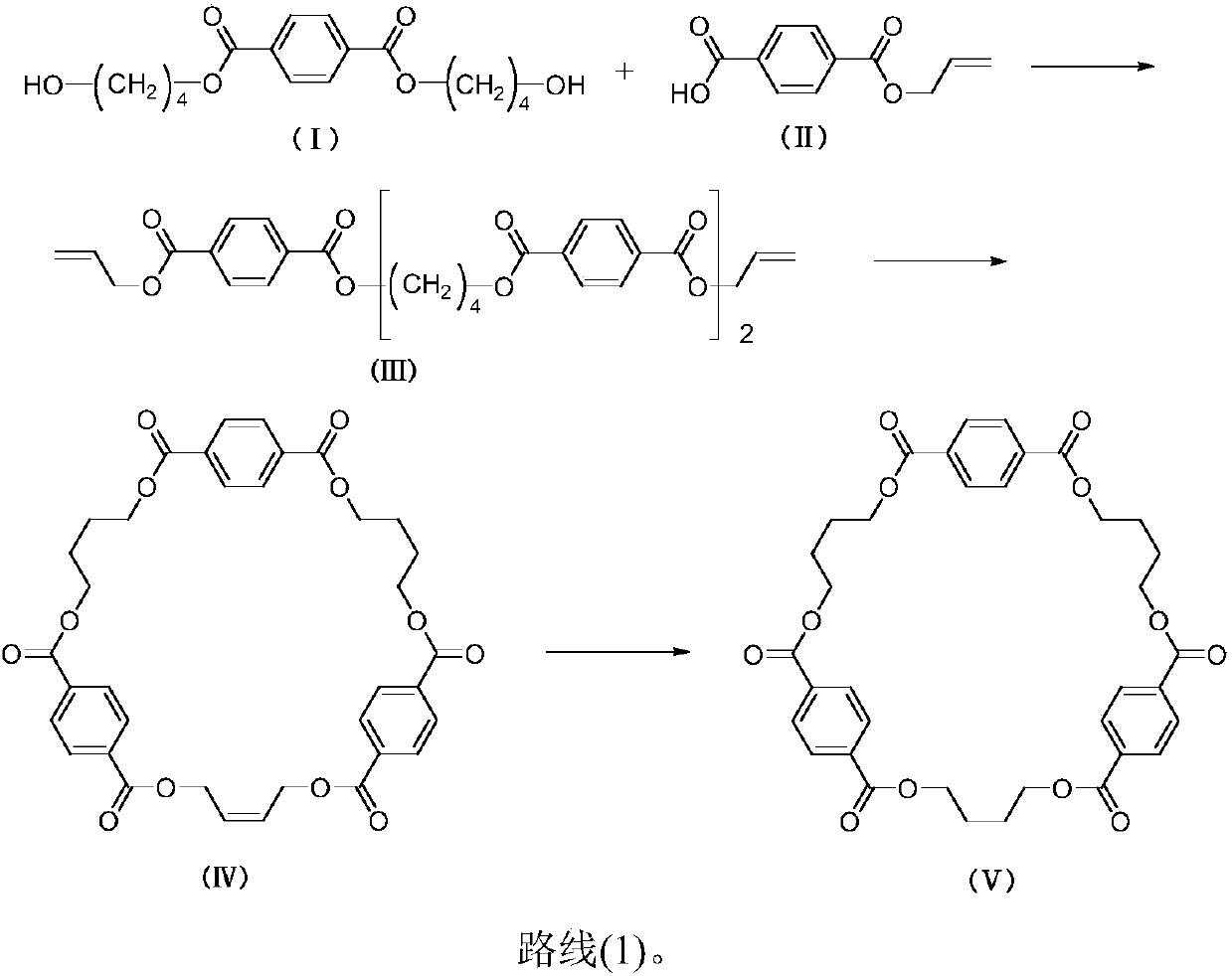
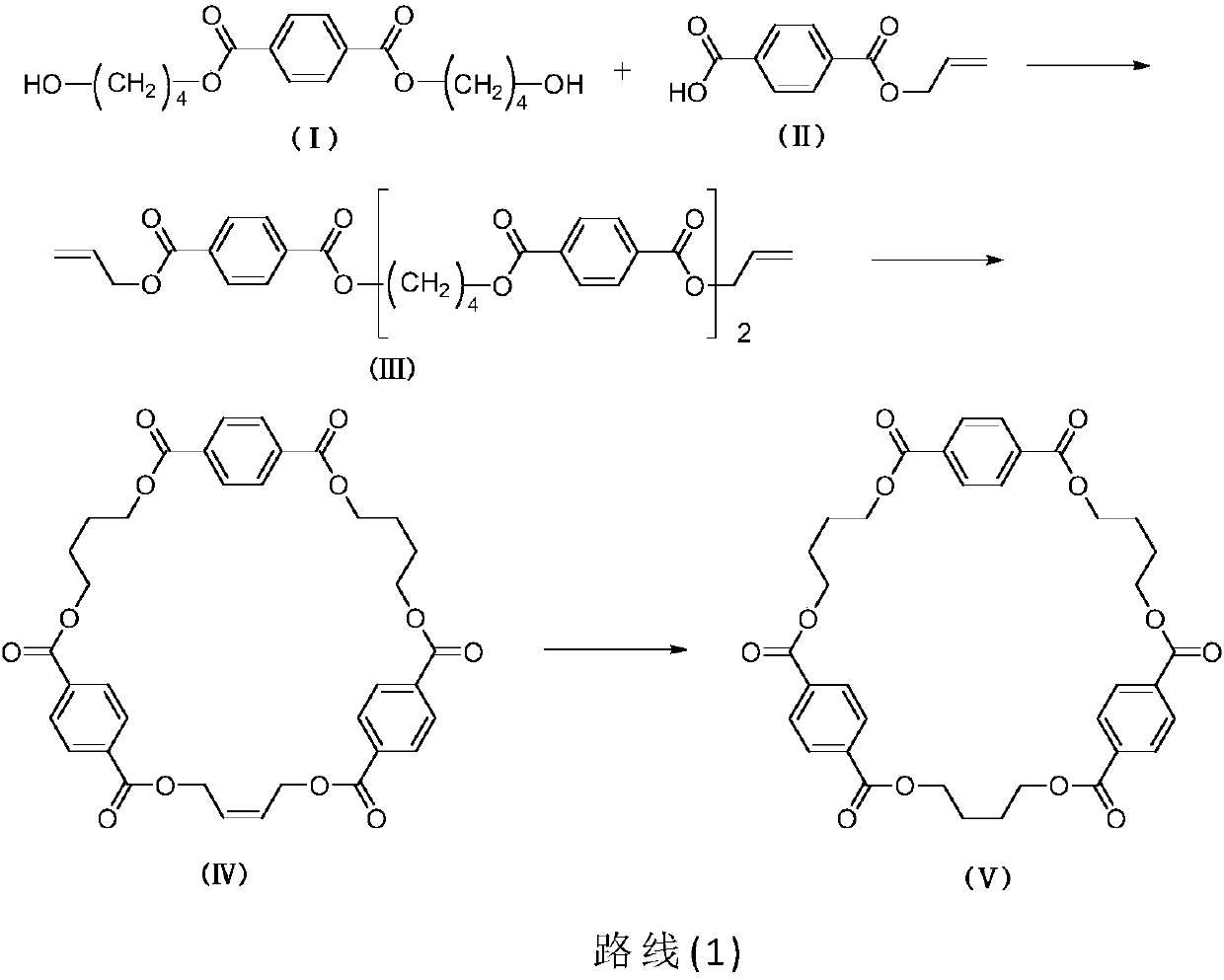
[0041] In a one-necked bottle, dissolve 1.43g of bis-(4-hydroxybutyl)-terephthalate and 1.57g of 4-(allyloxycarbonyl)benzoic acid in 20mL of chloroform, add 0.18g of DMAP, 2.33g of EDCI in sequence , heated to 85°C for reaction. After 24 hours of reaction, TLC detected that the reaction of raw materials was complete. Dilute hydrochloric acid was added for extraction, washed with water, the organic phase was separated, dried over anhydrous sodium sulfate, evaporated to dryness, and purified by column chromatography with chloroform. 1.58 g of (III) bis(4-(4-allylterephthaloyloxy)butyl)terephthalate was obtained in a yield of 75%.
[0042] 1 H NMR (400MHz, CDCl 3 )δ8.04(dd, J=9.3,6.9Hz,12H),5.98(ddd,J=22.5,10.9,5.7Hz,2H),5.42–5.20(m,4H),4.78(d,J=5.6Hz ,4H),4.37(s,8H),1.91(s,8H).
[0043] MS(ESI):m / z[M+Na] + :709.
[0044] In a single-necked bottle, 1.43g of bis-(4-hydroxybutyl)-terephthalate and 1.57g of 4-(allyloxycarbonyl)benzoic acid were dissolved in 20mL of chloroform...
Embodiment 2
[0051] Add 1.5g bis(4-(4-allyl terephthaloyloxy) butyl) terephthalate in the one-necked bottle, dissolve 0.75g Grubbs second-generation catalyst in chloroform, dropwise Then add 0.62g tetraisopropyl titanate and react at room temperature. The reaction was carried out for 12 hours, and TLC detected that the reaction of the raw materials was complete. The solid was removed by suction filtration, washed with water, dried, evaporated to dryness, and purified by column chromatography with a volume ratio of chloroform to ethyl acetate of 40:1. 0.94 g of the cyclic tripolybutylene terephthalate precursor of formula (IV) was obtained, and the yield was 65%.
[0052] 1 H NMR (400MHz, CDCl 3 )δ8.05(dd,J=9.0,6.8Hz,12H),5.98(s,2H),4.83(s,4H),4.36(s,8H),1.88(s,8H).
[0053] MS(ESI):m / z[M+Na] + :681.
[0054] Add 1.5g bis(4-(4-allyl terephthaloyloxy) butyl) terephthalate in the one-necked bottle, dissolve 0.75g Grubbs first-generation catalyst in chloroform, dropwise Then add 0.62 g ...
Embodiment 3
[0061] Dissolve 1.2 g of cyclic tripolybutylene terephthalate precursor in 20 mL of tetrahydrofuran in a one-necked bottle, add 0.24 g of palladium carbon, replace hydrogen, and react at room temperature. After 8 hours of reaction, TLC detected that the reaction of the raw materials was complete. The solid was removed by suction filtration, and the filtrate was evaporated to dryness and dried. 0.72 g of cyclic tripolybutylene terephthalate of formula (V) was obtained, and the yield was 95%.
[0062] 1 H NMR (400MHz, CDCl 3 )δ8.03(s,12H),4.36(s,12H),1.88(s,12H).
[0063] MS(ESI):m / z[M+Na] + :683.
[0064] Dissolve 1.2 g of cyclic tripolybutylene terephthalate precursor in 20 mL of tetrahydrofuran in a one-necked bottle, add 0.24 g of palladium carbon, replace hydrogen, and react at room temperature. React for 4 hours. The solid was removed by suction filtration, the filtrate was evaporated to dryness, and purified by beating with petroleum ether and ethyl acetate at a vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com