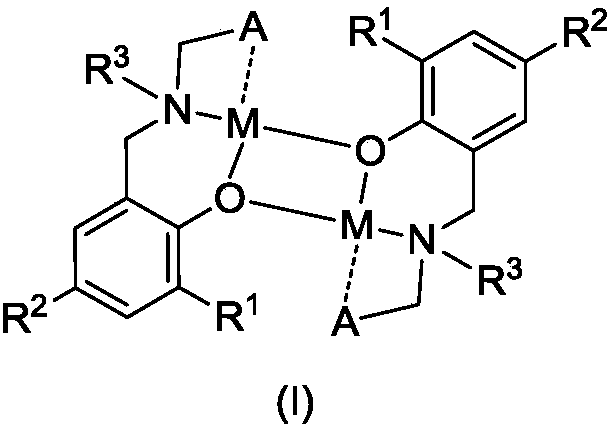
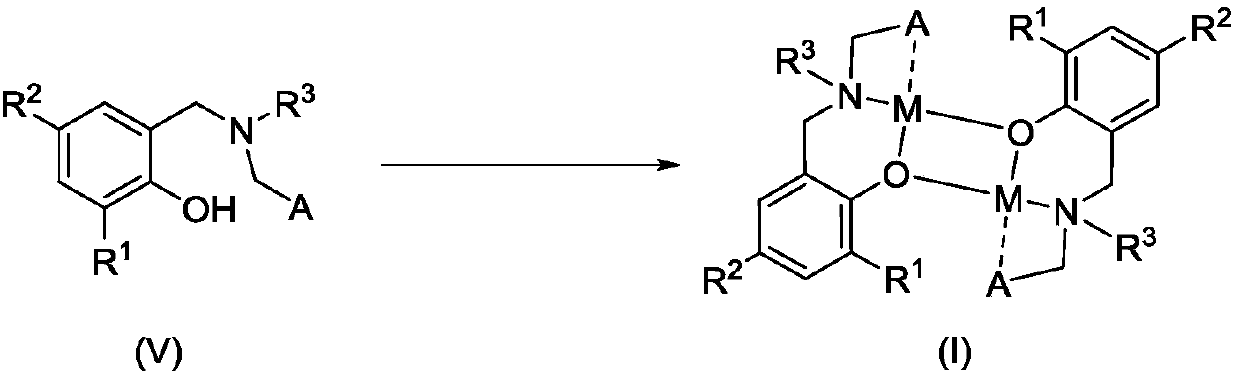
Amido phenoxy potassium complex, preparation method thereof and application of complex
A kind of amino phenoxy potassium and complex technology, which is applied in the field of amino phenoxy potassium complex, can solve the problem of low molecular weight of polymers, and achieves the effects of convenient preparation, wide application prospect and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Potassium Complex K1
[0047] Add Ligand L to a 50 mL Schlenk bottle 1 H (633mg, 1.00mmol), add 2mL of toluene to dissolve it, slowly drop KN(SiMe 3 ) 2 (200mg, 1.00mmol) in toluene (2mL), stirred overnight at room temperature. After filtration, the filtrate was drained to obtain a pale yellow foamy solid, which was recrystallized with a mixed solvent of toluene and n-hexane, and a pale yellow solid was precipitated, which was drained to obtain a pale yellow solid K1 (421 mg, 51%).
[0048]
[0049] 1 H NMR (C 6 D. 6 ,400MHz,298K):δ7.88–7.47(m,6H,ArH),7.27–7.20(m,2H,ArH),7.14–7.08(m,6H,ArH),7.07–7.03(m,2H,ArH ),7.03–6.94(m,5H,ArH),4.82(d,1H, 2 J=7.8Hz,CHN),4.59(pseudo-t,1H, 2 J≈ 3 J=7.0Hz,CH 2 O),4.29(d,1H, 2 J=10.0Hz, ArCH 2 ),3.26(d,1H, 2 J=10.0Hz, ArCH 2 ),3.04–2.84(m,3H,2H of NCH 2 C=N,1H of CHCH 2 ),2.83–2.73(m,2H,1Hof CHCH 2 ,1H of CH 2 CHCH 2 ),2.31(s,3H,ArCH 3 ),1.98–1.88(m,1H,CH 2 ofcyclohexyl), 1.74–1.64 (m, 1H, CH 2 of cy...
Embodiment 2
[0051] Synthesis of Potassium Complex K2
[0052] Ligand L 2 H (607mg, 1.00mmol), KN (SiMe 3 ) 2 (200mg, 1.00mmol), the rest of the steps are the same as in Example 1. K2 was obtained as a pale yellow solid (381 mg, 59%).
[0053]
[0054] 1 H NMR (C 6 D. 6 ,400MHz,298K):δ7.70–7.34(m,6H,ArH),7.27–7.17(m,2H,ArH),7.13–6.94(m,12H,ArH),6.92(m,1H, 3 J=8.0Hz, ArH), 5.20–4.86 (br, 1H, CHN), 4.64–4.56 (m, 1H, CHN) 2 O), 4.48–4.20 (br, 1H, ArCH 2 ), 3.50–3.04 (br, 1H, ArCH 2 ),3.04–2.63(m,4H,2HofNCH 2 C=N,2H of CHCH 2 ),2.62–2.42(br,1H,NCH 2),2.41–2.30(m,1H,NCH 2 ),2.23(s,3H,ArCH 3 ),1.36–1.15(m,2H,NCH 2 CH 2 ), 1.13–0.96 (m,2H,CH 2 CH 3 ),0.76(t,3H, 3 J=7.2Hz,CH 2 CH 3 ). 13 C{ 1 H}NMR (C 6 D. 6 ,100MHz,298K):δ167.8(OC=N),166.7,142.3,140.2,137.9,133.3,132.3,131.9,129.3,128.8,128.6,127.5,127.0,125.70,125.66,125.4,125.2,114 Ar-C), 82.7(CHN), 76.6(CHO), 64.3(Ph 3 C), 50.4 (ArCH 2 ), 39.6 (NCH 2 C=N),28.4(NCH 2 CH 2 ), 27.6 (PhCH 2 CH),21.46(NCH 2 CH 2 ...
Embodiment 3
[0056] Synthesis of Potassium Complex K3
[0057] Ligand L 3 H (607mg, 1.00mmol), KN (SiMe 3 ) 2 (200mg, 1.00mmol), the rest of the steps are the same as in Example 1. K3 was obtained as pale yellow solid (381 mg, 59%).
[0058]
[0059] 1 H NMR (C 6 D. 6 ,400MHz,298K):δ7.64–7.32(m,5H,ArH),7.20–7.16(m,3H,ArH),7.13–6.99(m,10H,ArH),6.98–6.90(m,4H,ArH ), 6.60 (s, 1H, ArH), 4.50–4.24 (br, 1H, CHN), 4.14–3.94 (br, 1H, CHN 2 O),3.68–3.20(br,3H,1H of CH 2 O,2H of ArCH 2 ), 3.06–2.74 (br, 3H, 2Hof NCH 2 C=N,1H of NCH 2 CH 2 ),2.68–2.43(br,2H,1H of NCH 2 CH 2 ,1H of PhCH 2 CH),2.39–2.29(m,1H,PhCH 2 CH),2.25(s,3H,ArCH 3 ), 1.45–1.30 (br, 2H, NCH 2 CH 2 ), 1.24–1.09 (m,2H,CH 2 CH 3 ),0.86(t,3H, 3 J=7.2Hz,CH 2 CH 3 ). 13 C NMR (C 6 D. 6 ,100MHz,298K):δ168.0(NCH 2 C=N), 166.7, 138.0, 137.9, 135.6, 133.4, 132.3, 129.5, 129.3, 129.0, 128.6, 127.0, 125.7, 125.0, 114.8 (All ArC), 71.1 (CHN), 67.4 (CH 2 O), 64.4 (Ph 3 C), 49.7 (ArCH 2 ), 41.9 (NCH 2 C=N),32.0(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com