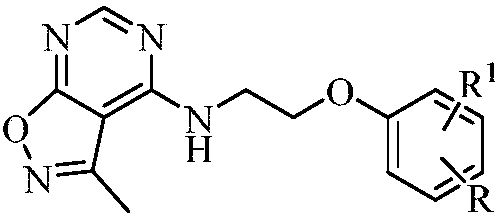
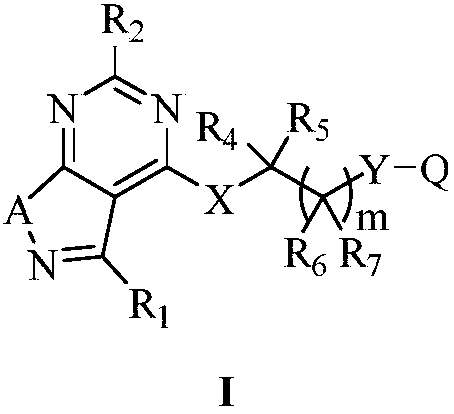
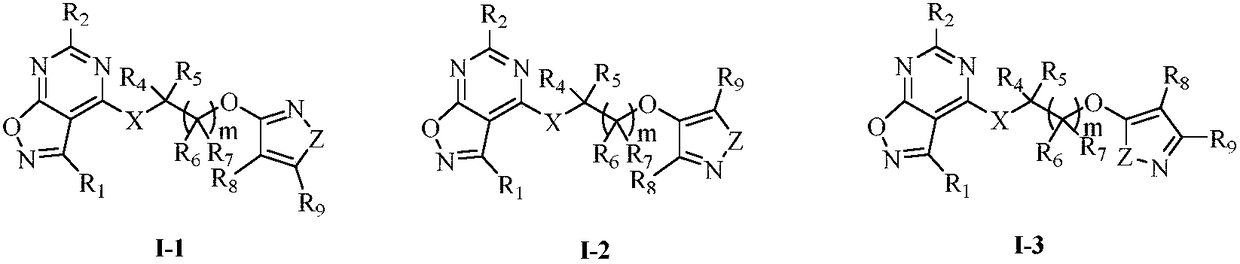
Substituted five-membered heterocyclic compound containing pyrimidine rings and preparation method and application thereof
A technology for five-membered heterocycles and compounds, which is applied to the field of substituted five-membered heterocycles containing pyrimido rings and the preparation thereof, and can solve the problems of very different structures and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0335] Embodiment 1: Preparation of intermediate 2-((3-methylisoxazolo[5,4-d]pyrimidin-4-yl)amino)ethanol
[0336] 1) Preparation of 5-amino-3-methylisoxazole-4-cyano
[0337]
[0338] Dissolve 14g (0.2 mol) of hydroxylamine hydrochloride in 80mL of 10% sodium hydroxide, add 27.2g (0.2 mol) of (1-ethoxyethylene) malononitrile, add a small amount of ice to keep the temperature below 50°C, and continue at room temperature After stirring for 1.5 hours, the solid was filtered, washed with water, and recrystallized from absolute ethanol to obtain 16.5 g of a solid product.
[0339] 2) Preparation of 4-chloro-3-methylisoxazolo[5,4-d]pyrimidine
[0340]
[0341] Phosphorus oxychloride (POCl 3 ) 40mL and N,N-dimethylformamide 1mL were added to the reaction flask, stirred at room temperature for 1 hour, added 5-amino-3-methylisoxazole-4-cyano group (451mg, 3.66mmol), and heated to React at 160°C for 15-36 hours, and distill the reaction solution under reduced pressure to obtai...
Embodiment 2
[0342] Embodiment 2: Synthesis of intermediate 2-(1-(4-chlorophenyl)-1H-pyrazole-3-oxyl)ethylamine hydrochloride
[0343] 1) Preparation of N-Boc-2-bromoethylamine
[0344]
[0345] Put 20.5g (0.1mol) of bromoethylamine bromate in 80ml of tetrahydrofuran, add 10.08g (0.12mol) of sodium bicarbonate and 50ml of water in sequence, and add 21.80g (0.1mol) of di-tert-butyl dicarbonate dropwise under stirring at room temperature Ester, after dropping, continue to react for 4-10 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, and (3×50ml) ethyl acetate was added for extraction. The organic phase was washed with 50ml of saturated brine, and 21.84g of a colorless oily liquid was obtained after precipitation, with a yield of 97.5%.
[0346] 2) Preparation of N-Boc-2-(1-(2-chlorophenyl)-1H-pyrazole-3-oxyl)ethylamine
[0347]
[0348] Mix 2.24g (0.01mol) N-Boc-2-bromoethylamine with 1.94g (0.01mol) 1-(4-chlorophenyl)-1H-3-hydroxypyrazole...
Embodiment 3
[0352] Embodiment 3: the preparation of compound 21-180
[0353]
[0354] 0.17g (0.001mol) of 4-chloro-3-methylisoxazolo[5,4-d]pyrimidine and 0.27g (0.001mol) of 2-(1-(4-chlorophenyl)-1H-pyridine Azole-3-oxy)ethylamine hydrochloride was added to 50ml of toluene. Add 0.45g (0.002mol) triethylamine, heat to reflux, and react for 4-10 hours. After the reaction is monitored by TLC, evaporate the solvent under reduced pressure, add (3×30ml) ethyl acetate for extraction, and use saturated brine for the organic phase After washing with 50ml, the residue was subjected to column chromatography (eluent: ethyl acetate and petroleum ether (boiling range: 60-90°C), volume ratio: 1:4) to obtain 0.27g of a white solid. The melting point is 156.5°C.
[0355] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3)δ(ppm):2.46(3H,s),4.06-4.12(2H,q),4.55-4.58(2H,t),5.93(1H,d),5.94(2H,m),7.37(2H,dd ), 7.51(2H,dd), 7.72(1H,d), 8.51(1H,s).
[0356] Other compounds of the present invention c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com