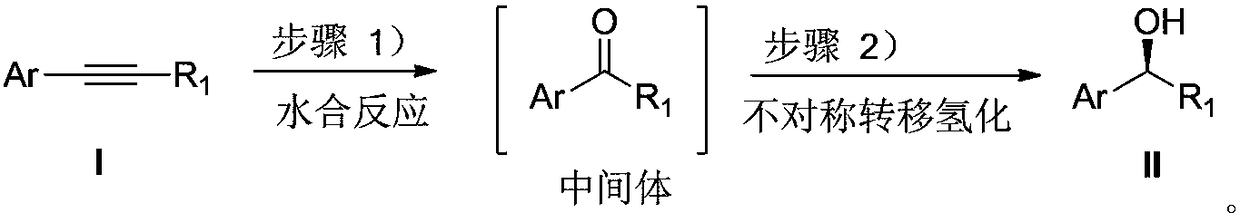
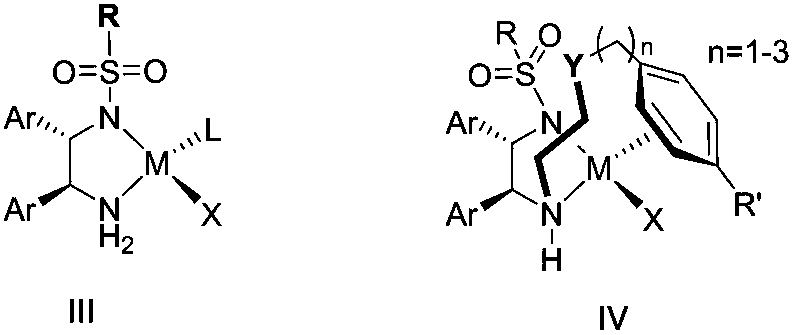
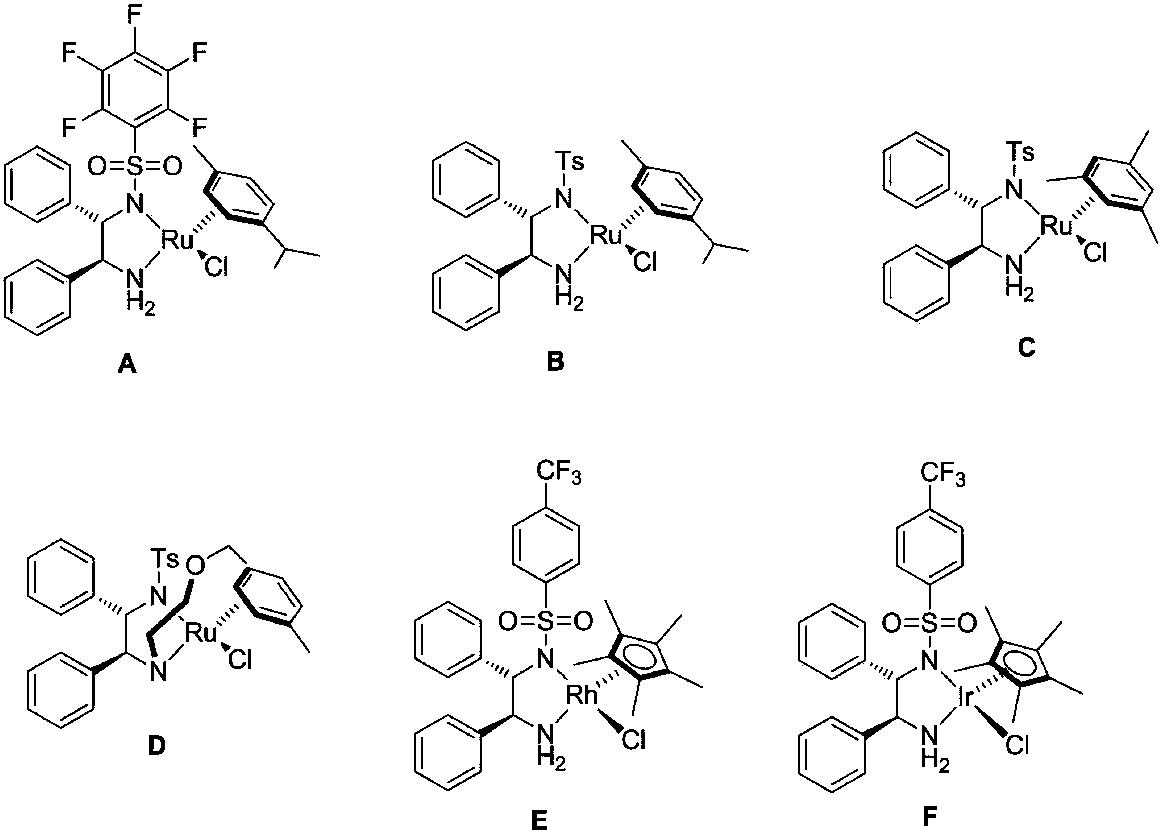
Method for direct conversion of aromatic alkyne into chiral alcohol through one-pot process
A technology for conversion into and chiral alcohols, applied in organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of complex synthesis of ketones and organometallic reagents, difficult to obtain, etc., and achieves easy operation and reaction conditions. Mild, enantioselective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the asymmetric synthesis of (S)-1-phenylethanol
[0033]
[0034] Add 0.5 mmol of phenylacetylene to the test tube, followed by adding CF 3 SO 3 H (20mol%, 9uL), H 2 O(2equiv., 20 uL), CF 3 CH 2 OH (1mL), after reacting at 40°C for 4h, add 0.005mmol catalyst A, HCOONa (0.5mmol, 34mg, 2.5mmol, 170mg), H 2 O (1 mL), react at 50°C for 5 hours. After the reaction was finished, it was extracted 3 times with ethyl acetate, and the combined organic phases were concentrated to dryness. The separation yield: 93% (petroleum ether: ethyl acetate=5:1), and the ee of the product (S)-1-phenylethanol was determined by HPLC. The value is 97%. HPLC separation conditions: chiral column Daicel OD-H-H column, mobile phase: n-hexane / isopropanol=97:3 (volume ratio), flow rate: 1.0 ml / min, wavelength: 254 nm, column temperature: 30 degrees Celsius, t 1 = 11.58 minutes, t 2 = 13.82 minutes; 1 H NMR (400MHz, CDCl 3 ):δ=7.43-7.37(m,4H),7.34-7.30(m,1H),4.93(dd,J 1 =12...
Embodiment 2
[0035] Embodiment 2: the asymmetric synthesis of (S)-1-phenylethanol
[0036]
[0037] Add 0.5 mmol of phenylacetylene to the test tube, followed by adding CF 3 SO 3 H (20mol%, 9uL), H 2 O(2equiv., 20 uL), CF 3 CH 2 OH (1mL), after reacting at 40°C for 4h, add 0.005mmol catalyst B, HCOONa (0.5mmol, 34 mg), H 2O (1 mL), react at 50°C for 5 hours. After the reaction was finished, it was extracted 3 times with ethyl acetate, and the combined organic phases were concentrated to dryness. The isolated yield: 41% (petroleum ether: ethyl acetate=5:1), and the ee of the product (S)-1-phenylethanol was determined by HPLC. The value is 93%.
Embodiment 3
[0038] Embodiment 3: the asymmetric synthesis of (S)-1-phenylethanol
[0039]
[0040] Add 0.5 mmol of phenylacetylene to the test tube, followed by adding CF 3 SO 3 H (20mol%, 9uL), H 2 O(2equiv., 20 uL), CF 3 CH 2 OH (1mL), after reacting at 40°C for 4h, add 0.005mmol catalyst D, HCOONa (0.5mmol, 34 mg), H 2 O (1 mL), react at 50°C for 5 hours. After the reaction was finished, it was extracted 3 times with ethyl acetate, and the combined organic phases were concentrated to dryness. The isolated yield: 68% (petroleum ether: ethyl acetate=5:1), and the ee of the product (S)-1-phenylethanol was determined by HPLC. value of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com