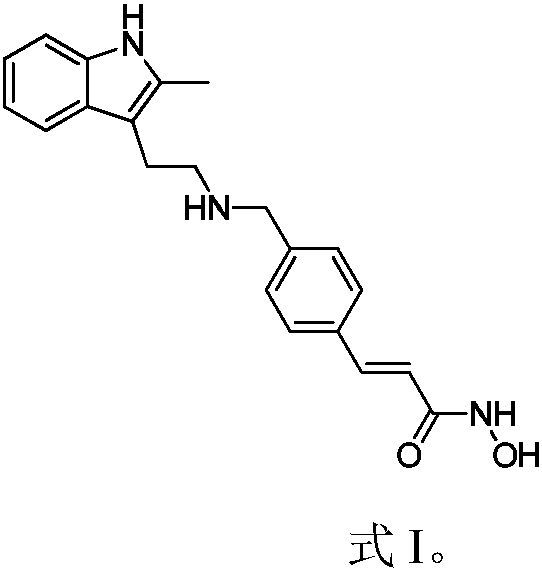
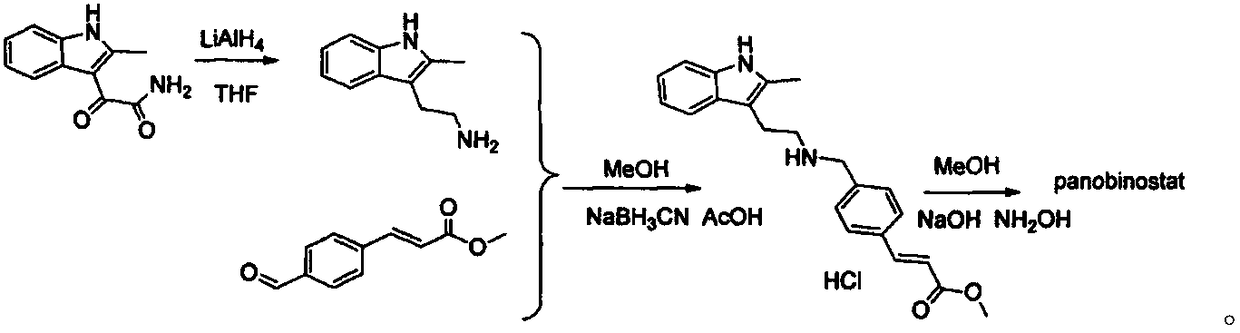
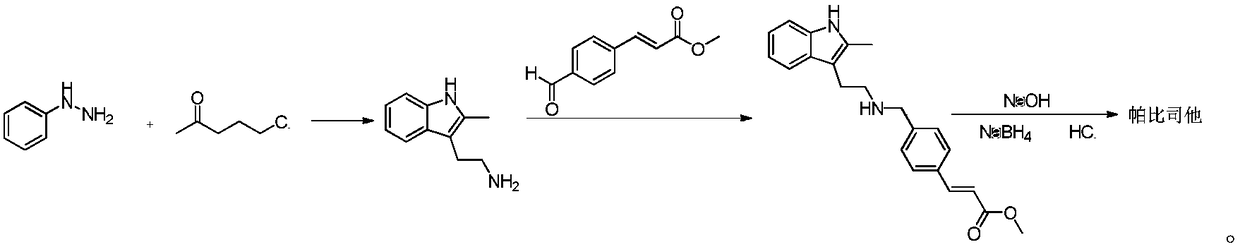
Synthesis method of panobinostat key intermediate
A synthetic method, panobinostat technology, applied in the field of drug synthesis, can solve the problems of low purity and yield of intermediates, difficulty in quality control of panobinostat raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 (E)-3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]methyl acrylate hydrochloride preparation
[0023] Weigh 5.0g 2-methyltryptamine and 5.75g (E)-3-(4-formylphenyl)methyl acrylate into the reaction flask, add 50ml methanol to dissolve, stir at room temperature for 1h, add 0.05g palladium carbon , Continue to stir for 4h under hydrogen atmosphere at room temperature, the reaction is over, celite is filtered, the solvent is evaporated under reduced pressure to obtain (E)-3-[4-[[[2-(2-methyl-1H-indole-3) -Yl)ethyl]amino]methyl]phenyl]methyl acrylate crude.
[0024] Weigh 4.5 g of the obtained crude (E)-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]methacrylate. In 10ml methanol, add 2.4g hydrogen chloride methanol solution dropwise, stir for 0.5h at room temperature, add 5ml acetone, 1ml water, and stand for 8h at 0-4℃ to obtain 5.53g of the title compound, yield 90%, purity 99.8% by HPLC .
Embodiment 2
[0025] Example 2 (E)-3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]methyl acrylate hydrochloride preparation
[0026] Weigh 5.0g 2-methyltryptamine and 5.75g (E)-3-(4-formylphenyl)methyl acrylate into the reaction flask, add 50ml methanol to dissolve, stir at room temperature for 1h, add 0.25g palladium carbon , Continue to stir for 4h under hydrogen atmosphere at room temperature, the reaction is over, celite is filtered, the solvent is evaporated under reduced pressure to obtain (E)-3-[4-[[[2-(2-methyl-1H-indole-3) -Yl)ethyl]amino]methyl]phenyl]methyl acrylate crude.
[0027] Weigh 3.0 g of the obtained crude (E)-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]methacrylate. In 12ml of ethanol, add 1.6g of hydrogen chloride methanol solution dropwise, stir at room temperature for 0.5h, add 5ml of acetone, 1ml of water, and place for 8h at 0-4℃ to obtain 3.1g of the title compound, with a yield of 88% and a purity of 99.8% by HPLC .
Embodiment 3
[0028] Example 3 (E)-3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]methyl acrylate hydrochloride preparation
[0029] Weigh 5.0g 2-methyltryptamine and 5.75g (E)-3-(4-formylphenyl)methyl acrylate into the reaction flask, add 50ml methanol to dissolve, stir at room temperature for 1h, add 0.25g palladium carbon , Continue to stir for 4h under hydrogen atmosphere at room temperature, the reaction is over, celite is filtered, the solvent is evaporated under reduced pressure to obtain (E)-3-[4-[[[2-(2-methyl-1H-indole-3) -Yl)ethyl]amino]methyl]phenyl]methyl acrylate crude.
[0030] Weigh 3.0 g of the obtained crude (E)-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]methacrylate. In 10ml methanol, add 1.6g hydrogen chloride methanol solution dropwise, stir at room temperature for 0.5h, add 5ml methyl ethyl ketone, 1ml water, and stand for 8h at 0-4℃ to obtain 3.55g of the title compound, yield 93%, purity 99.5% by HPLC .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com