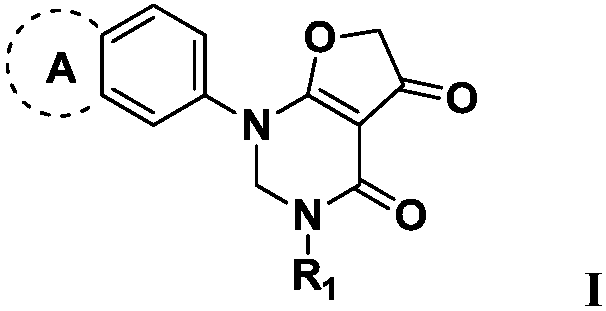
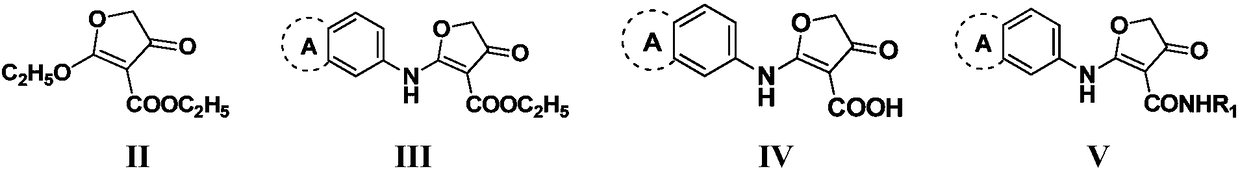
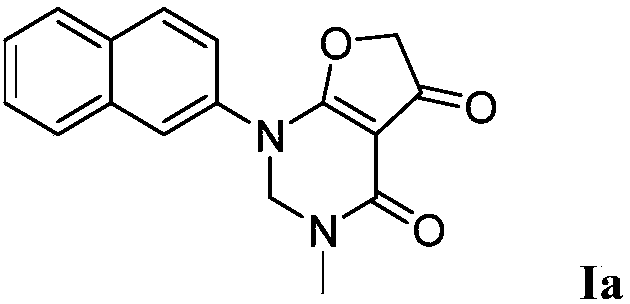
Pyrimidone derivative and application thereof
A derivative, pyrimidinone technology, applied in the direction of drug combination, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of poor selectivity, easy to produce drug resistance, etc., and achieve the effect of significant activity inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 3-methyl-1-(naphthalene-2-yl)-2,3-dihydro[2,3-d]pyrimidine-4,5(1H,6H)-dione (compound represented by formula Ia)
[0030]
[0031] (1) Synthesis of ethyl 2-(2-naphthylamino)-4-carbonyl-4,5-dihydrofuran-3-carboxylate (compound shown in formula IIIa):
[0032]
[0033] Add 3mmol of sodium hydrogen (60% purity) and 1.8mL of anhydrous tetrahydrofuran (THF) into a 50mL flask, add 6mmol of diethyl malonate in 3mL of anhydrous THF dropwise in an ice-water bath, and then Add 3mmol of chloroacetyl chloride in 3mL of anhydrous THF dropwise, and stir at 40°C to 45°C for at least 1 hour;
[0034] At room temperature, add 2-naphthylamine dropwise to the above reaction solution, stir at 45°C to 55°C for at least 12 hours, adjust the pH of the reaction solution to 6-7 with 5% dilute hydrochloric acid solution, and use ethyl acetate to Extraction, washing with saturated brine twice, concentration of the organic layer, drying, silica gel column chromatography (petrol...
Embodiment 2
[0051] 1-(2-naphthyl)-3-ethyl-2,3-dihydrofuro[2,3-d]pyrimidine-4,5-(1H, 6H)-dione (compound shown in formula Ib) synthesis:
[0052]
[0053] Except that the ethylamine aqueous solution was used to replace the methylamine aqueous solution in Example 1 (in step (3)), other conditions and steps were the same as in Example 1 to obtain a white solid (compound represented by formula Ib) with a yield of 37.3%.
[0054] 1 H NMR (400MHz, CDCl 3 ):δ8.03-7.73(m,4H),7.60-7.42(m,3H),5.51(s,2H),4.88(s,2H),3.05(q,J=7.6 3H),1.33(t, J=7.6 3H).
[0055] 13 C NMR (100MHz, CDCl 3 )δ193.6, 182.4, 162.3, 135.4, 133.3, 132.3, 131.8, 128.4, 128.2, 127.5, 127.2, 124.2, 123.6, 97.6, 38.6, 38.5, 14.9.
[0056] LC-MS (ESI) calcd for C 18 h 16 N 2 o 3 [M+H] + 309.02, found 309.09.
Embodiment 3
[0058] 1-(2,3-dihydro-1H-inden-5-yl)-3-ethyl-2,3-dihydrofuro[2,3-d]pyrimidine-4.5(1H,6H)-dione ( Compound shown in formula Ic) synthesis:
[0059]
[0060] Except replacing 2-naphthylamine in Example 1 (in step (1)) with the compound shown in formula c, and replacing methylamine aqueous solution in Example 1 with ethylamine aqueous solution (in step (3)), other conditions and steps Same as Example 1, a white solid (compound represented by formula Ic) was obtained with a yield of 39.1%.
[0061] 1 H NMR (400MHz, CDCl 3 )δ7.32(d, J=8.0Hz, 1H), 7.27(s, 1H), 7.16(d, J=8.0Hz, 1H), 4.22(q, J=7.2Hz, 2H), 4.17(s, 2H), 3.65(s, 2H), 2.89(t, J=7.6Hz, 4H), 2.09-2.02(m, 2H), 1.26(t, J=7.2Hz, 3H).
[0062] 13 C NMR (100MHz, CDCl 3 )δ190.9, 183.5, 165.6, 145.7, 144.2, 135.8, 125.4, 123.6, 121.6, 97.1, 88.3, 59.7, 38.4, 32.8, 32.4, 25.7, 14.9.
[0063] LC-MS (ESI) calcd for C 17 h 18 N 2 o 3 [M+H] + 299.13, found 299.17.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com