no n -Application of acyl amino acid ester in the preparation of antitumor drugs
A technology of acyl aminoester and anti-tumor drugs, applied in the field of anti-tumor drugs and drugs, to achieve the effects of high encapsulation efficiency, good biocompatibility and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
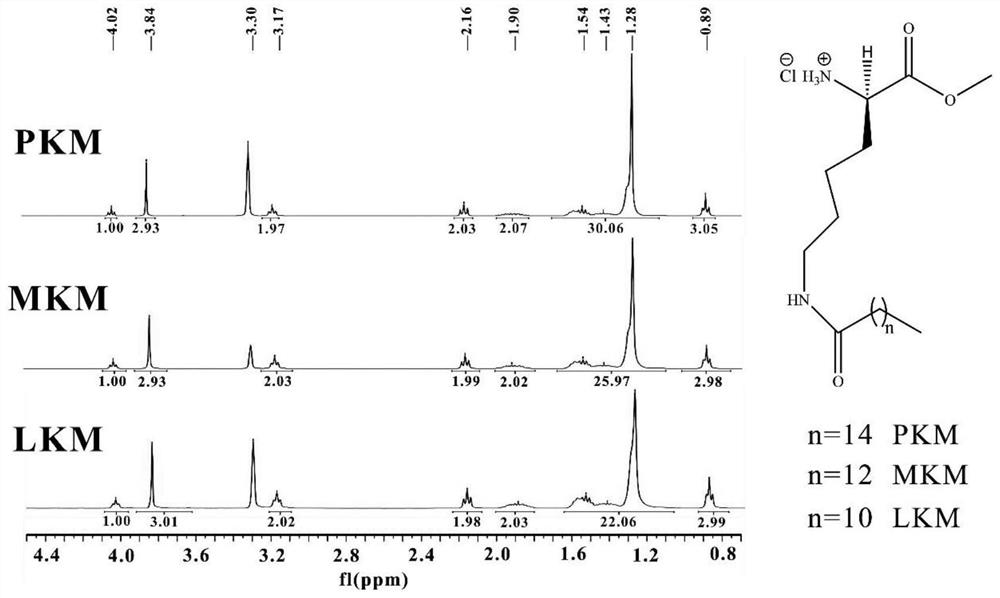
[0042] Preparation example: the synthetic method of AKE (formula 1-A):
[0043] (1) Esterification reaction
[0044] Will N α -Benzyloxycarbonyllysine (N α -Cbz-lys, the lysine whose α-amino group is protected by benzyloxycarbonyl) and the corresponding alcohol (R2-OH) (per 1mmol N α -Cbz-lys, add 3-10ml R2-OH), stir and cool in -10℃ ethanol bath for 10-30min, add thionyl chloride dropwise (every 1mmol N α -Cbz-lys was added with 5-7 mmol thionyl chloride), and stirring was continued at room temperature for 20 h. Evaporate under reduced pressure to remove excess thionyl chloride, methanol and HCl generated in the reaction until a white solid is obtained, which is dried in vacuo at 50°C to obtain the esterification reaction product N α -Benzyloxycarbonyl lysine ester (N α -Cbz-lysine ester).
[0045] (2) Acylation reaction
[0046] The last step esterification reaction product N α -Cbz-lysine ester, ethyl acetate and triethylamine (add 20-30ml of methanol and 3-4mmol of...
Embodiment 1
[0052] Embodiment 1 liposome preparation method
[0053] The preparation method of the folic acid modified targeted liposome (FA-PEG-AKE-LP) of the medicine of the present invention:
[0054]Prepared by conventional film hydration method, soybean lecithin (SPC), drug AKE (MKM was specifically used in this case), polyethylene glycol 2000-distearoylphosphatidylethanolamine (PEG2000-DSPE), folic acid-polyethylene Diol 2000-distearoylphosphatidylethanolamine (FA-PEG2000-DSPE) is added into the eggplant-shaped bottle at a molar ratio of 60:20:5:0.5, and fully dissolved with 50-100 times (w / w) methanol , use a rotary evaporator to evaporate under reduced pressure in a 45°C water bath to form a thin film in the bottle, and then use a vacuum pump to completely remove methanol. Then add 50-100 times (w / w) 0.01M pH7.4 phosphate buffered saline solution (PBS) to the eggplant-shaped bottle, use a rotary evaporator to hydrate in a water bath at 45°C for 30 minutes to obtain a milky white ...
Embodiment 2
[0057] Drug encapsulation efficiency and drug loading stability test of embodiment 2 liposome
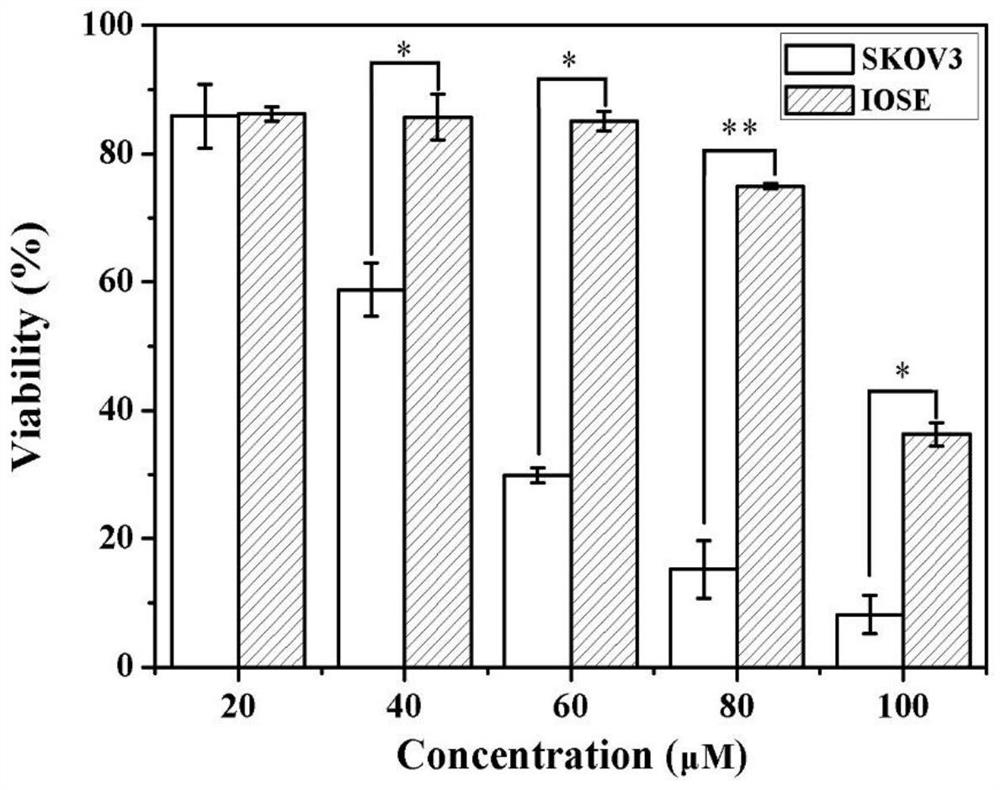
[0058] Drug Encapsulation Efficiency Determination After the drug-loaded liposome stock solution is prepared, take a sample in an ultrafiltration tube (30KD), centrifuge for 10min under 12000g centrifugal force with a desktop high-speed centrifuge, and obtain the centrifugate with a high-performance liquid chromatography (HPLC). Analyze free drug concentration. By comparing with the dosage, the drug encapsulation efficiency can be calculated. Results The encapsulation efficiency of the drug AKE of the present invention in the drug-loaded liposome (prepared in Example 1) was 92.6%-93.7%, indicating that the encapsulation efficiency of the drug in the liposome was relatively high (over 90%).
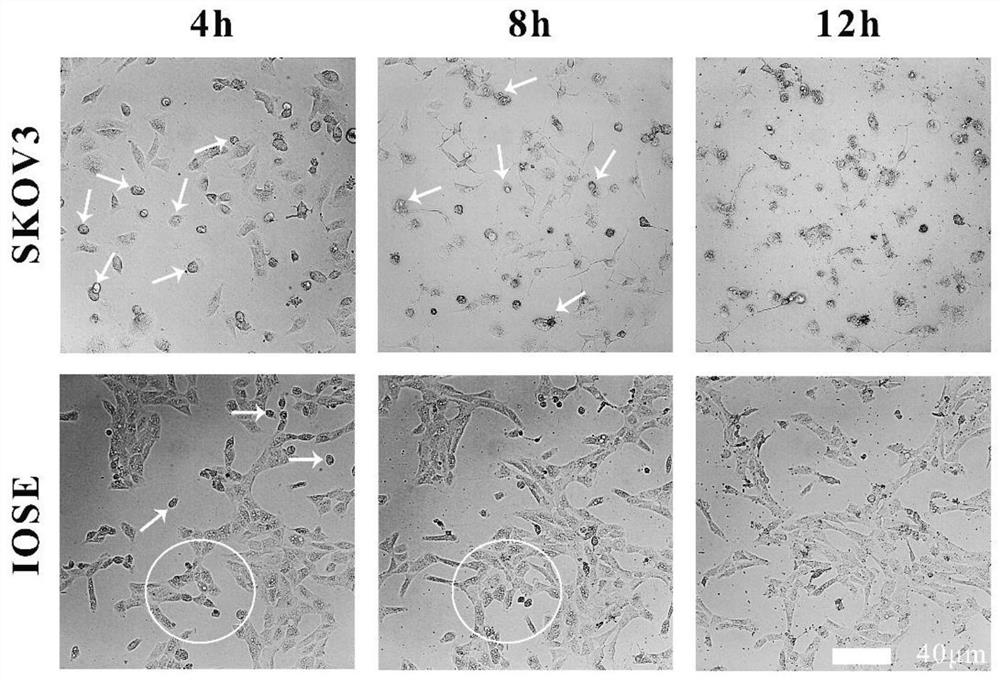
[0059] Drug-loaded stability test After the drug-loaded liposome stock solution is prepared, it is diluted with 0.01M PBS with pH 7.4 and complete cell culture medium containing serum to a conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com