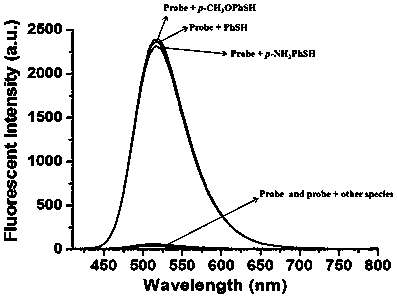
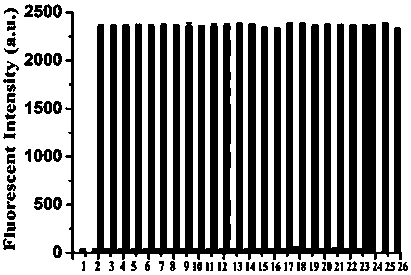
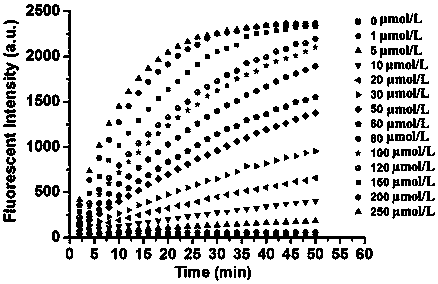
Coumarin thiophenol fluorescent probes and preparation method thereof
A fluorescent probe, coumarin technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as combustion and explosion, and achieve the effect of easy separation and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Synthesis of probes. Dissolve 50.0mg (0.20 mmol) of 2,3,6,7-tetrahydro-10-hydroxy-1H,5H-quinazino(9,1-GH)coumarin in 3ml of dichloromethane, add 58.0mg (0.40 mmol) K 2 CO 3 , 37.2 mg (0.20 mmol) of 2,4-dinitrofluorobenzene, stirred at room temperature, TLC followed the reaction, the reaction was complete in 12 hours, extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, filtered, evaporated under reduced pressure Solvent, separated and purified by silica gel column chromatography to obtain 62.3 dark red solids with a yield of 73.6%. 1 H NMR (400 MHz, CDCl 3 ): δ 8.86(s, 1 H), 8.32 (d, J = 6.8 Hz, 1 H), 7.53(s, 1 H), 7.10 (d, J = 9.2 Hz, 1 H), 6.88 (s, 1 H), 3.29-3.33 (m, 4 H), 2.77-2.90 (m, 4 H), 1.99-2.09 (m, 4 H). 13 C NMR (100 MHz, CDCl 3 ): Δ 157.13, 155.44,150.25, 146.30, 141.65, 138.64, 133.16, 131.39, 128.69, 125.10, 122.25,119.76, 117.64, 106.90, 49.65, 27.53, 21.25, 20.36. HRMS (ESI) (C) 13 h 15 NO 3 ) m...
Embodiment 2
[0017] Example 2: Synthesis of probes. Dissolve 50.0mg (0.20 mmol) of 2,3,6,7-tetrahydro-10-hydroxy-1H,5H-quinazino(9,1-GH)coumarin in 3mL of dichloromethane, add 0.06mL (0.40 mmol) Et 3 N, 37.2 mg (0.20 mmol) 2,4-dinitrofluorobenzene, stirred at room temperature, TLC followed the reaction, the reaction was complete in 10 hours, extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, filtered, evaporated under reduced pressure The solvent was removed and purified by silica gel column chromatography to obtain 73.9 mg of dark red solid with a yield of 87.4%.
Embodiment 3
[0018] Example 3: Synthesis of probes. Dissolve 50.0mg (0.20 mmol) of 2,3,6,7-tetrahydro-10-hydroxyl-1H,5H-quinazino(9,1-GH)coumarin in 3ml of acetonitrile, add 0.09mL (0.60 mmol) Et 3 N, 37.2mg (0.20mmol) 2,4-dinitrofluorobenzene, stirred at room temperature, TLC followed the reaction, the reaction was complete in 8 hours, extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, filtered, evaporated under reduced pressure The solvent was removed and purified by silica gel column chromatography to obtain 69.5 mg of reddish-brown solid with a yield of 82.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com