A di -hydrogen bayonin pharmaceutical and preparation method based on TWEEN80 and chitosan as the carrier
A technology of dihydromyricetin and chitosan, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor solubility and limit practical application, and achieve improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
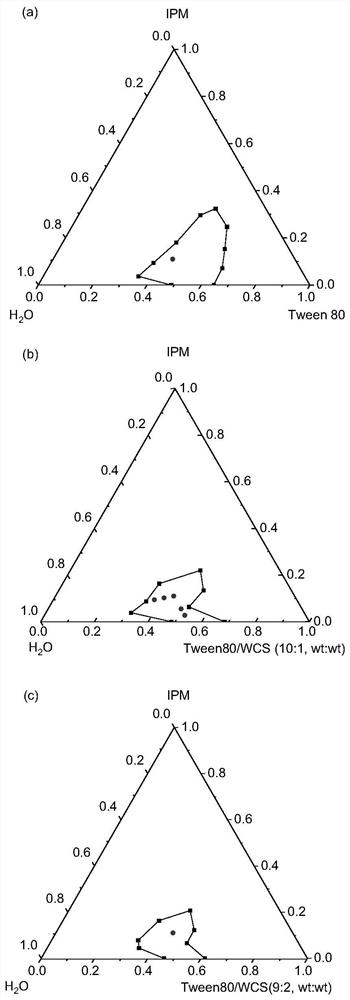
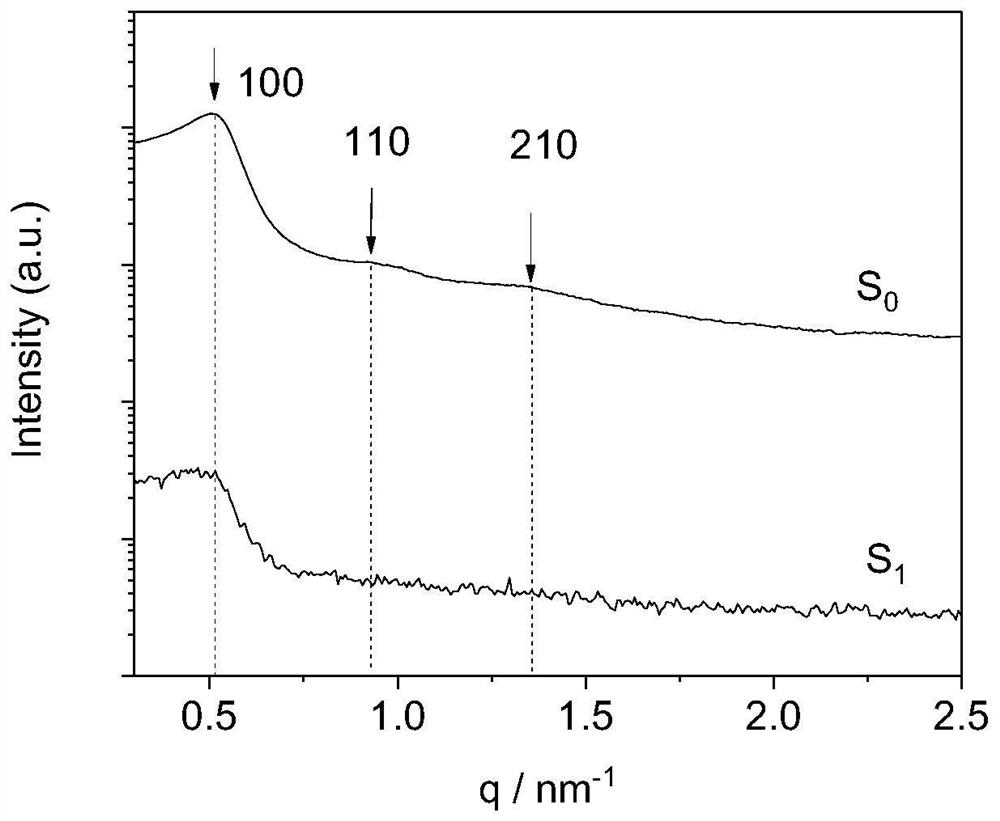
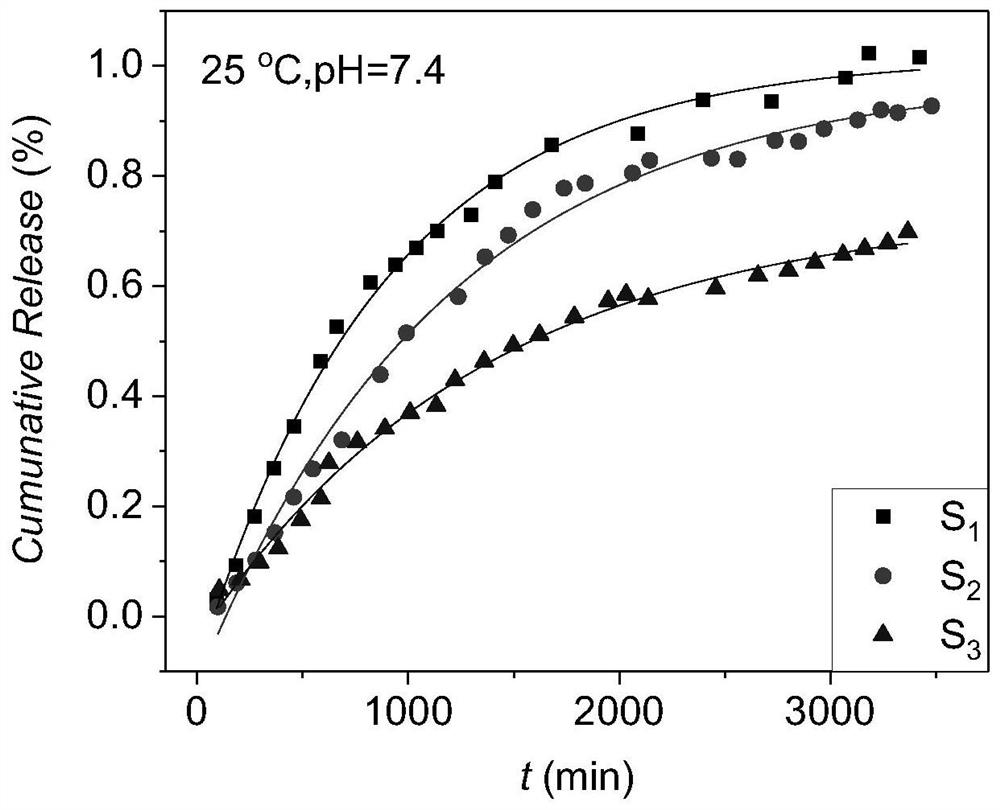
[0050] Surfactant / oil / water ratio is 44 / 11 / 45, and chitosan mass fraction is 4%, weighs Tween 80 and WCS (as surfactant) and places in colorimetric tube, mixes and makes chitosan Disperse evenly in Tween 80. IPM was added thereto, and stirred and mixed evenly under a water bath at 65°C. Finally, add double-distilled water dropwise to the colorimetric tube, the water content increases at intervals of 2%, stir evenly with a magnetic stirrer, then place it in a water bath at 25°C to balance, observe and record the phase state and appearance of the aggregate The change of the aggregate needs to prolong the equilibrium time of the aggregate when approaching the phase boundary. The phase boundary is preliminarily judged by visually observing the color, transparency, hardness, viscosity, etc. of the aggregates. denoted as S 1
Embodiment 2
[0052] Surfactant / oil / water ratio is 44 / 11 / 45, and chitosan mass fraction is 8%, weighs Tween 80 and WCS (as surfactant) and places in colorimetric tube, mixes and makes chitosan Disperse evenly in Tween 80. IPM was added thereto, and stirred and mixed evenly under a water bath at 65°C. Finally, add double-distilled water dropwise to the colorimetric tube, the water content increases at intervals of 2%, stir evenly with a magnetic stirrer, then place it in a water bath at 25°C to balance, observe and record the phase state and appearance of the aggregate The change of the aggregate needs to prolong the equilibrium time of the aggregate when approaching the phase boundary. The phase boundary is preliminarily judged by visually observing the color, transparency, hardness, viscosity, etc. of the aggregates. denoted as S 2 .
Embodiment 3
[0056] The ratio of surfactant to oil is 9:1, the mass fraction of chitosan is 4.5%, and the water content is 45%. Weigh Tween80 and WCS (as surfactant) and place them in colorimetric tubes, mix well to make the shell Glycans are evenly dispersed in Tween 80. IPM was added thereto, and stirred and mixed evenly under a water bath at 65°C. Finally, add double-distilled water dropwise to the colorimetric tube, the water content increases at intervals of 2%, stir evenly with a magnetic stirrer, then place it in a water bath at 25°C to balance, observe and record the phase state and appearance of the aggregate The change of the aggregate needs to prolong the equilibrium time of the aggregate when approaching the phase boundary. The phase boundary is preliminarily judged by visually observing the color, transparency, hardness, viscosity, etc. of the aggregates. denoted as S 1 o 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com