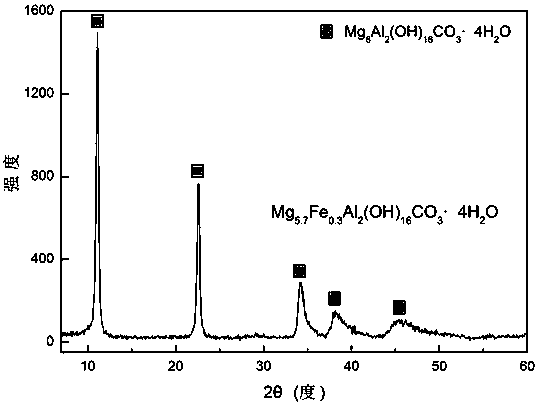
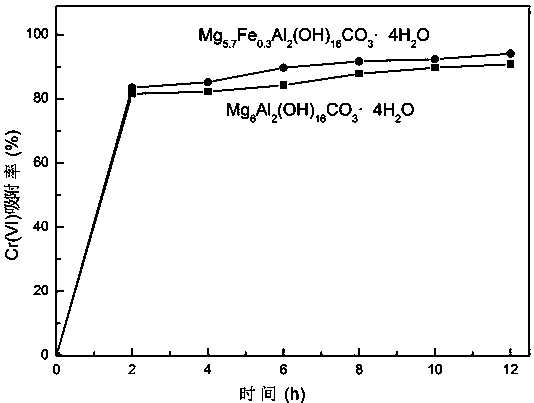
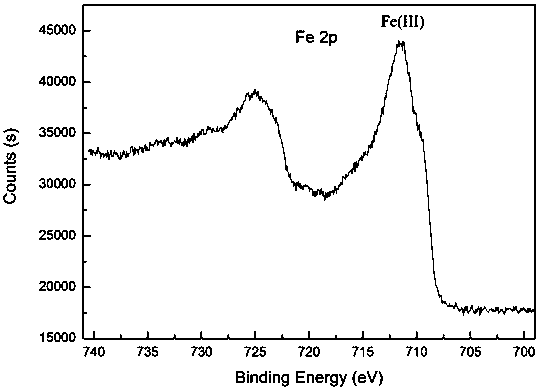
Method for adsorbing and reducing Cr (VI) based on iron magnesium aluminum hydrotalcite
A technology of hydrothermal method and iron-containing raw materials, which is applied in the direction of chemical instruments and methods, reduced water/sewage treatment, water pollutants, etc., can solve the problems of secondary pollution, Cr(VI cannot be fundamentally removed, etc., and avoid The effect of secondary pollution, low raw material and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis:
[0042] 1) Press Mg 5.7 Fe 0.3 al 2 (OH) 16 CO 3 4H 2 The stoichiometric ratio of O weighs raw material magnesium nitrate (Mg(NO 3 ) 2 ) 1.6872g, ferrous nitrate (Fe(NO 3 ) 2 ) 0.1079g, aluminum nitrate (Al(NO 3 ) 3 9H 2 O) 1.5005g, sodium hydroxide (NaOH) 1.28g and sodium carbonate (NaOH) 2 CO 3 ) 0.212g, in Mg(NO 3 ) 2 , Fe(NO 3 ) 2 , Al(NO 3 ) 3 9H 2 O was added deionized water to form a homogeneous solution A, in NaOH and Na 2 CO 3 Add deionized water to form a homogeneous solution B.
[0043] 2) Add solution A to solution B dropwise under magnetic stirring, and pour the resulting mixed suspension into an autoclave, preheat at 60°C for 1 hour, then raise to 120°C for 15 hours of heat treatment. Finally, after washing, suction filtration and drying, Mg 5.7 Fe 0.3 al 2 (OH) 16 CO 3 4H 2 O sample;
[0044] 3) Add the sample to the prepared 6 groups of potassium dichromate solutions with a concentration of 80 mg / L. After fully s...
Embodiment 2
[0047] Synthesis:
[0048] 1) Press Mg 5.4 Fe 0.6 al 2 (OH) 16 CO 3 4H 2 The stoichiometric ratio of O weighs the raw material magnesium chloride (MgCl 2 ) 1.0283g, ferrous chloride (FeCl 2 ) 0.1521g, aluminum chloride (AlCl 3 ) 0.5334g, potassium hydroxide (KOH) 1.7952g and potassium carbonate (K 2 CO 3 ) 0.2764g, in MgCl 2 , FeCl 2 , AlCl 3 Add deionized water to form a homogeneous solution A, in KOH and K 2 CO 3 Add deionized water to form a homogeneous solution B.
[0049] 2) Add solution A to solution B dropwise under magnetic stirring, and pour the resulting mixed suspension into an autoclave, preheat at 70°C for 1 hour, then raise to 180°C for 18 hours of heat treatment. Finally, after washing, suction filtration and drying, Mg 5.4 Fe 0.6 al 2 (OH) 16 CO 3 4H 2 O sample;
[0050] 3) Add the sample to the prepared 6 groups of potassium dichromate solutions with a concentration of 90 mg / L. After fully stirring, take the supernatant of one group at in...
Embodiment 3
[0053] Synthesis:
[0054] 1) Press Mg 4.8 Fe 1.2 Al 2 (OH) 16 CO 3 4H 2 The stoichiometric ratio of O weighs raw material magnesium nitrate (Mg(NO 3 ) 2 ) 1.4208g, ferrous chloride (FeCl 2 ) 0.3042g, aluminum nitrate (Al(NO 3 ) 3 9H 2 O) 1.5005g, sodium hydroxide (NaOH) 1.28g and potassium carbonate (K 2 CO 3 ) 0.2764g, in Mg(NO 3 ) 2 , FeCl 2 , Al(NO 3 ) 3 9H 2 Add deionized water to O to form a homogeneous solution A, in NaOH and K 2 CO 3 Add deionized water to form a homogeneous solution B.
[0055] 2) Add solution A to solution B dropwise under magnetic stirring, and pour the resulting mixed suspension into an autoclave, preheat at 80°C for 1 hour, then raise to 150°C for 12 hours of heat treatment. Finally, after washing, suction filtration and drying, Mg 4.8 Fe 1.2 Al 2 (OH) 16 CO 3 4H 2 O sample;
[0056] 3) Add the sample to the prepared 6 groups of potassium dichromate solutions with a concentration of 100 mg / L. After fully stirring, take ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com