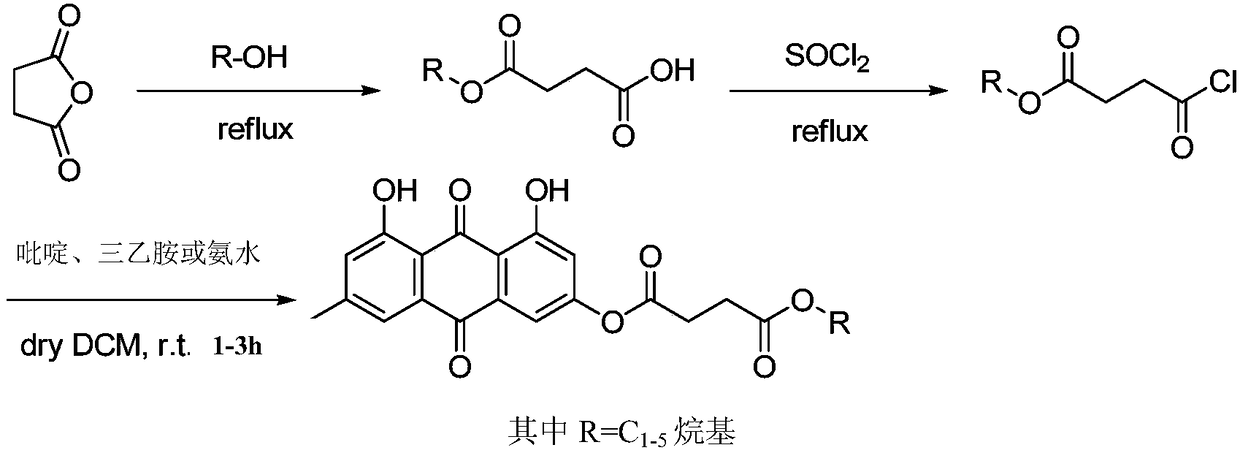
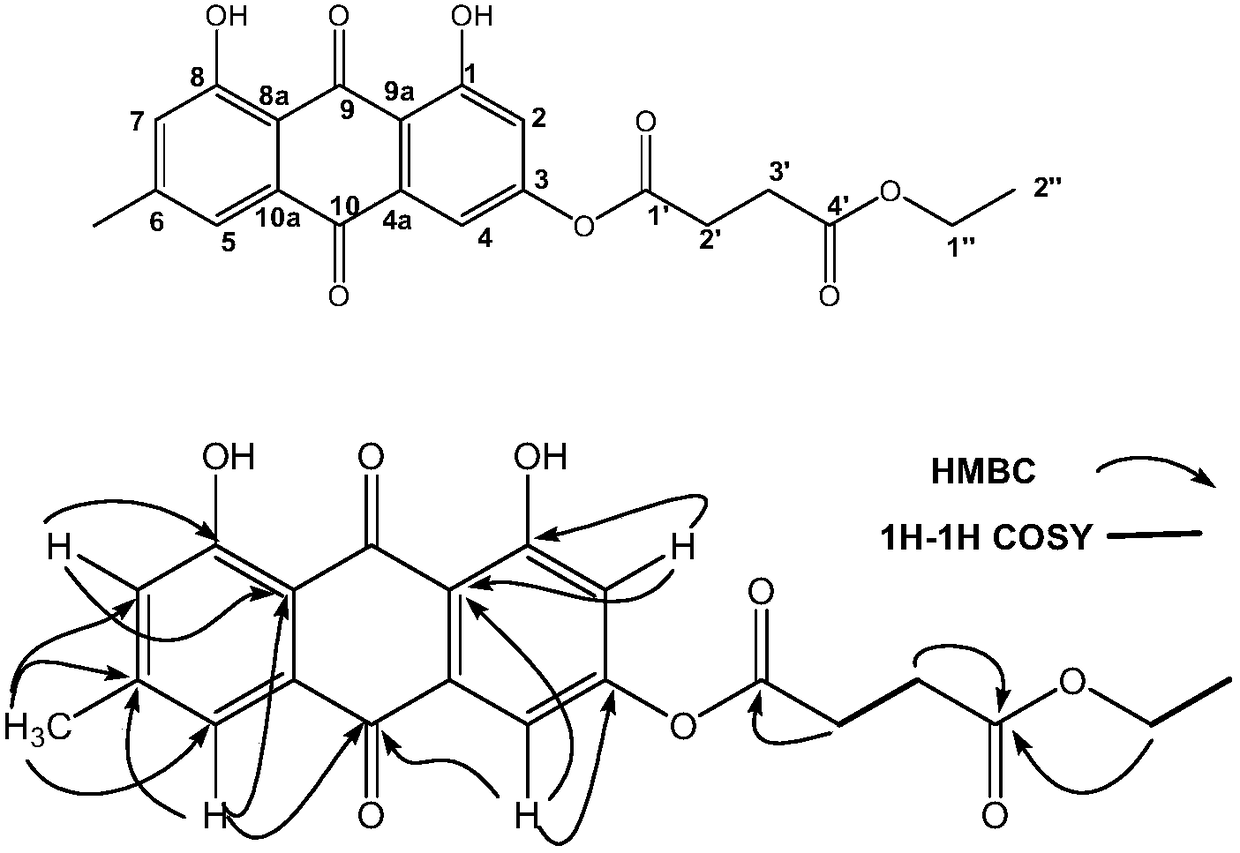
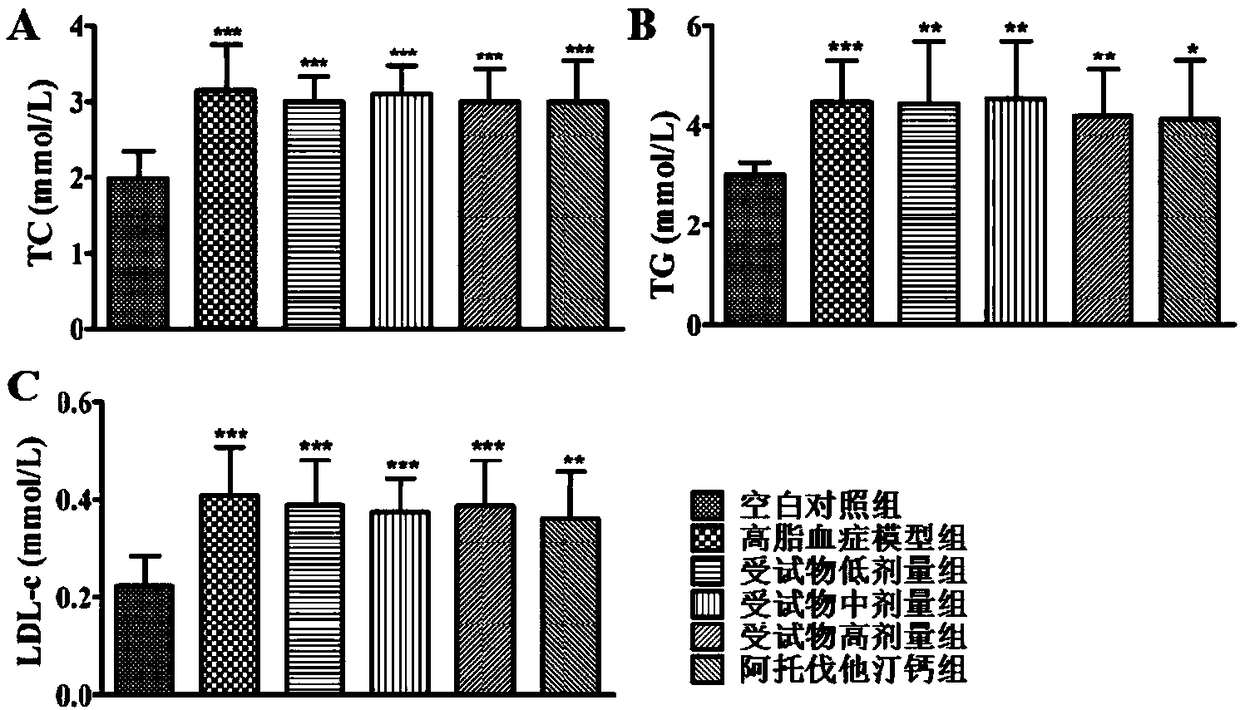
Applications of emodin succinyl ester compound in preparing lipid-lowering medicines
A technology of emodin succinyl ester and emodin succinyl ethyl ester, which is applied in the field of medicine, can solve the problems of liver tissue cell biochemical changes, cardiac rhabdomyolysis, and restriction of long-term medication, and achieve safe and effective hyperlipidemia, oral administration Absorption, the effect that facilitates the absorption of organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 emodin ethyl succinate
[0055] Take succinic anhydride (1.0g, 10mmol) and place it in a 10mL round-bottomed flask, use ethanol (3.5mL, 60mmol) as a solvent, heat and reflux for 4 hours, and distill off excess ethanol under reduced pressure to obtain a light yellow oil, namely succinic acid mono Ethyl ester (1.4 g, 96%), this product was carried on to the next reaction without isolation.
[0056] Get monoethyl succinate (1.0g, 6.8mmol) and place in a 10mL round-bottomed flask, use thionyl chloride (4.0g, 34.0mmol) as solvent, heat to reflux for 2 hours, and remove excess oxychloride by distillation under reduced pressure. The sulfone was obtained as a light yellow oily product, namely ethyl succinic acid chloride (1.1 g, 98%), and the product was directly carried on to the next reaction without separation.
[0057] Take emodin (1.0g, 3.7mmol) and pyridine (0.45g, 5.6mmol) in a 10mL round-bottomed flask, use dichloromethane (3mL) as solve...
Embodiment 2
[0062] The preparation of embodiment 2 emodin ethyl succinate
[0063] Take succinic anhydride (10g, 100mmol) and place it in a 150mL round-bottomed flask, use ethanol (40mL, 600mmol) as a solvent, heat and reflux for 6 hours, and distill off excess ethanol under reduced pressure to obtain a light yellow oil that is monoethyl succinate (14 g, 96%), the product was directly carried on to the next reaction without isolation.
[0064] Get monoethyl succinate (10g, 68mmol) and place in a 150mL round-bottomed flask, use thionyl chloride (40g, 340mmol) as a solvent, heat and reflux for 2 hours, and remove excess thionyl chloride by distillation under reduced pressure to obtain light The yellow oil was succinic acid monoethyl chloride (11 g, 98%), and the product was directly carried on to the next reaction without isolation.
[0065] Get emodin (10g, 37mmol) and triethylamine (2.3g, 22mmol) and place in a 250mL round-bottomed flask, use dichloromethane (3mL) as solvent, slowly drop...
Embodiment 3
[0068] The preparation of embodiment 3 emodin ethyl succinate
[0069] Take succinic anhydride (10g, 100mmol) and place it in a 150mL round-bottomed flask, use ethanol (20mL, 434mmol) as a solvent, heat and reflux for 2 hours, and distill off excess ethanol under reduced pressure to obtain a light yellow oil that is monoethyl succinate (14 g, 96%), the product was directly carried on to the next reaction without isolation.
[0070] Get monoethyl succinate (10g, 68mmol) and place in a 150mL round-bottomed flask, use thionyl chloride (20g, 170mmol) as a solvent, heat and reflux for 1 hour, and remove excess thionyl chloride by distillation under reduced pressure to obtain light The yellow oil was succinic acid monoethyl chloride (8 g, 98%), and the product was directly carried on to the next reaction without isolation.
[0071] Take emodin (10g, 37mmol) and ammonia water (1.5g, 44mmol) and place in a 250mL round-bottomed flask, use dichloromethane (3mL) as solvent, slowly add s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com