Novel selenization method for imidazopyridine derivative C3 position
A technology of imidazopyridine and its derivatives, which is applied in the fields of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of insufficient range of substrates, insufficient range of reaction types, high reaction temperature, etc., and achieve good compatibility, The steps are simple and easy to operate, and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
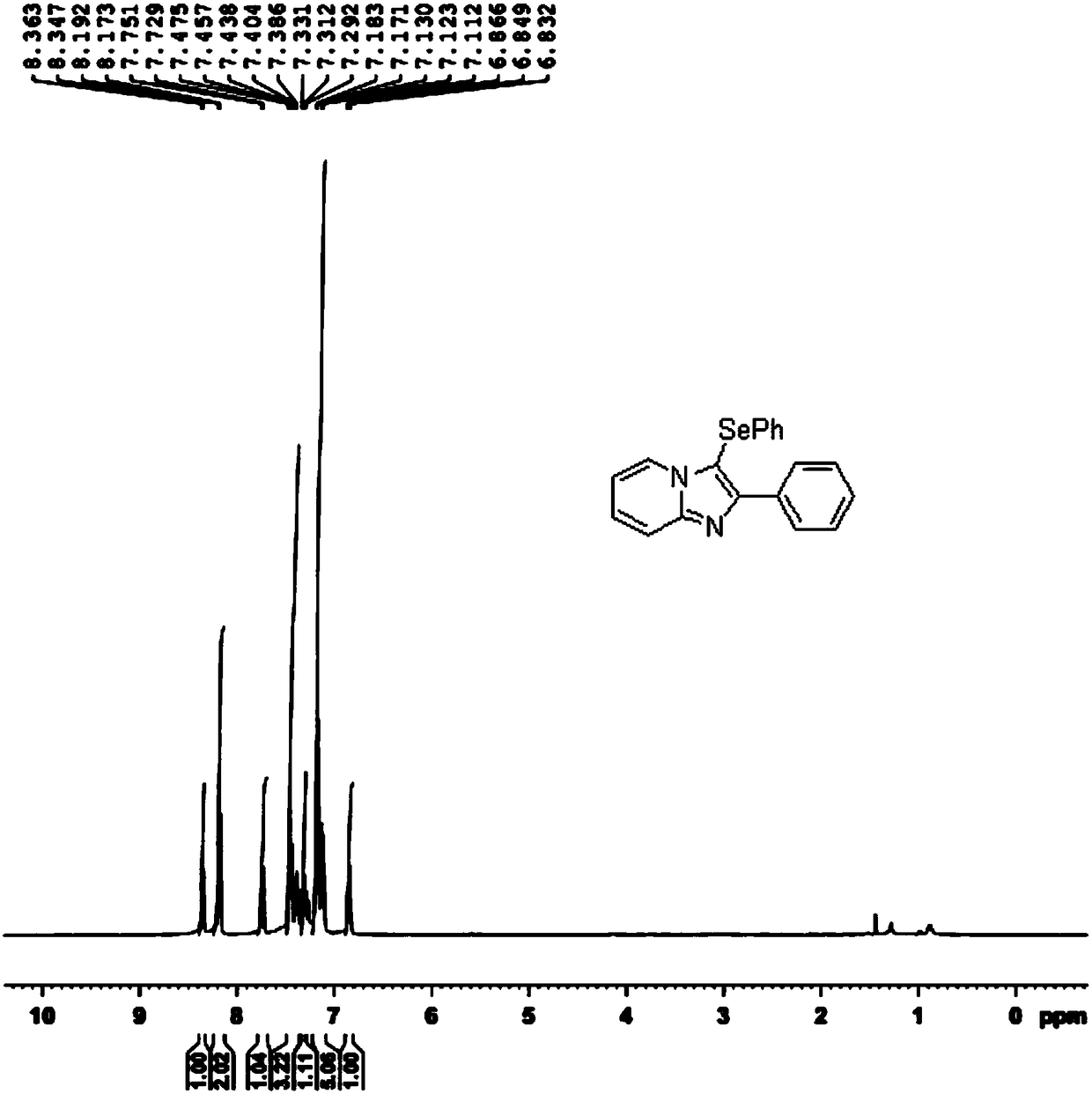
[0029] In a 50mL round bottom flask, add 0.1941g imidazopyridine, 0.3139g diphenyl diselenide, 0.2358g elemental iodine, 0.2120g potassium carbonate, 6.0mL dichloroethane (DCE), heat at 85°C, and react for 6h to The imidazopyridine reaction was complete (monitored by thin layer chromatography TLC). The reaction mixture was poured into 20 mL of water and extracted with ethyl acetate (10 mL×5). The organic phases were combined and dried with anhydrous sodium sulfate; after the solvent was evaporated, the residue was separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 6 / 1) to obtain a white solid imidazopyridine derivative at position C3 The selenization product was 0.3083g, and the yield was 88%.
[0030] The specific response is shown in the following formula:
[0031]
[0032] The 1H NMR spectrum of the selenization product at the C3 position of the imidazopyridine derivative is as follows figure 1 As shown, the spectrogram analysis data is as f...
Embodiment 2
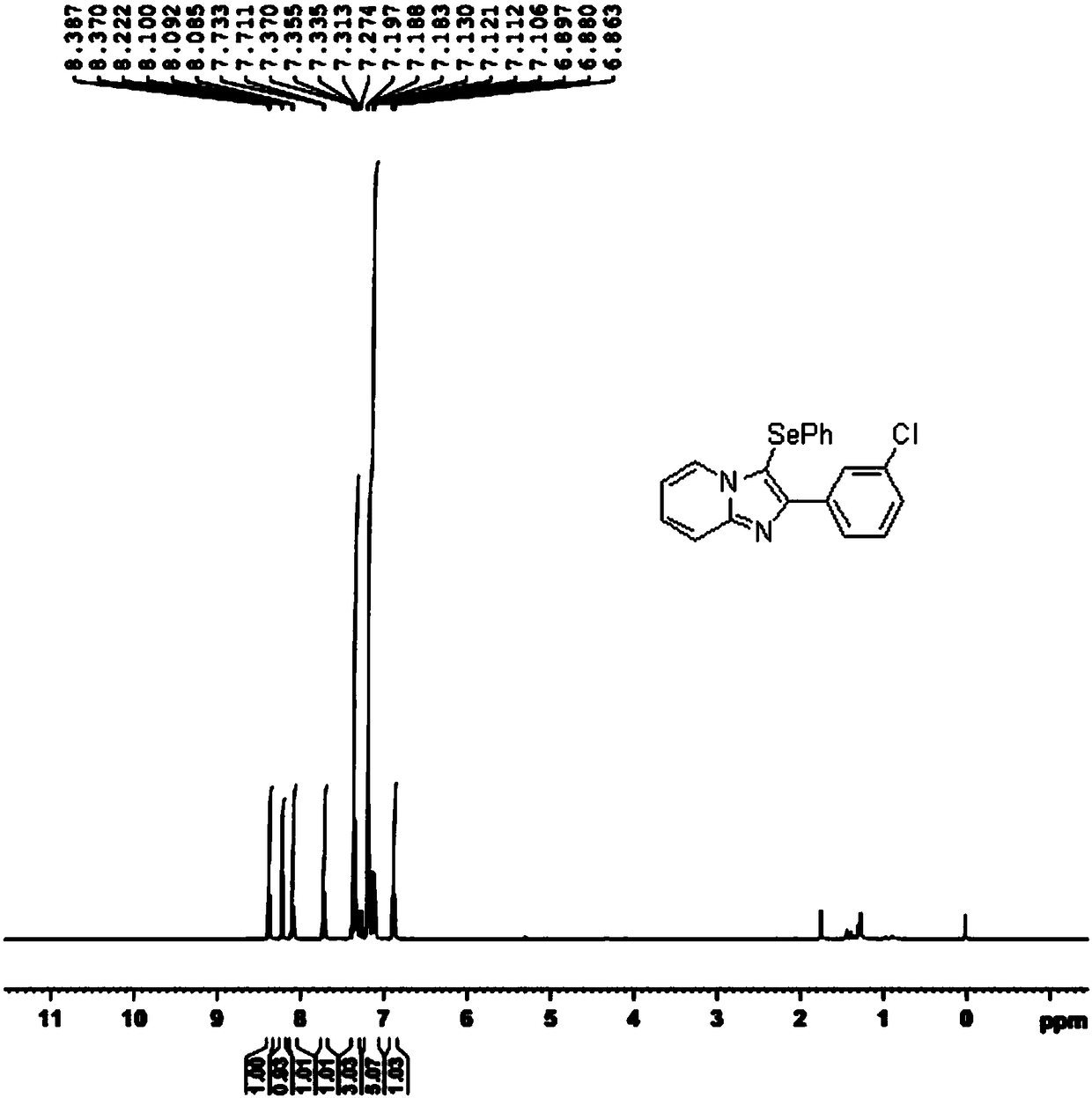
[0035] In a 50mL round bottom flask, add 0.2280g 3-chlorophenylimidazopyridine, 0.3139g diphenyl diselenide, 0.2358g elemental iodine, 0.2120g potassium carbonate, 6.0mL dichloroethane (DCE), heat 85 At °C, the reaction was conducted for 6 hours until the imidazopyridine reaction was completed (monitored by thin layer chromatography TLC). The reaction mixture was poured into 20 mL of water, extracted with ethyl acetate (10 mL×5), and the organic phases were combined and dried with anhydrous sodium sulfate; after the solvent was evaporated, the residue was separated by silica gel column chromatography (eluent: petroleum ether / Ethyl acetate=6 / 1) to obtain 0.3456 g of the selenization product at the C3 position of the white solid imidazopyridine derivative, with a yield of 90%.
[0036] The specific response is shown in the following formula:
[0037]
[0038] The 1H NMR spectrum of the selenization product at the C3 position of the imidazopyridine derivative is as follows figure 2...
Embodiment 3
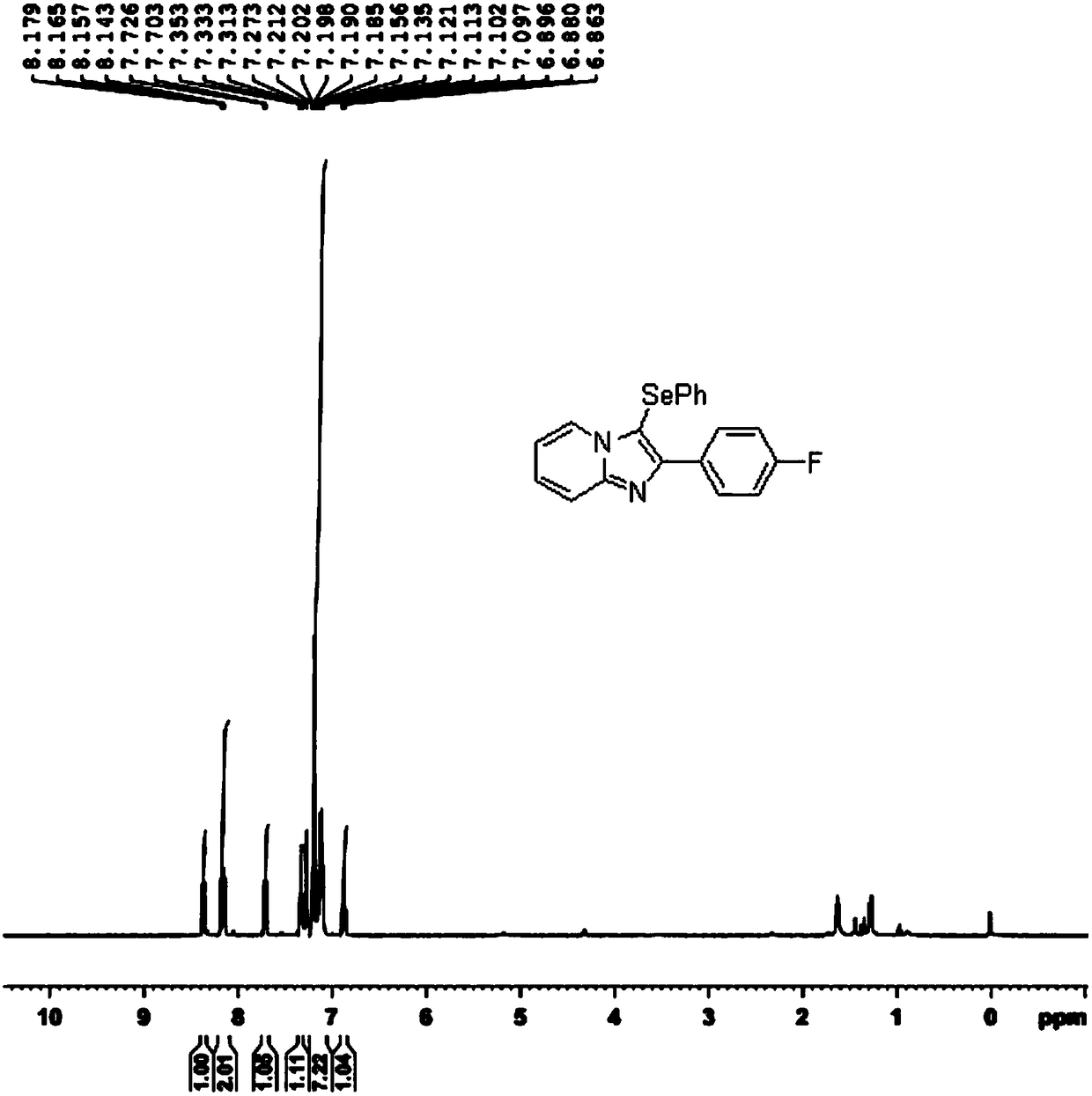
[0041] In a 50mL round bottom flask, add 0.2121g 4-fluorophenylimidazopyridine, 0.3139g diphenyl diselenide, 0.2358g elemental iodine, 0.2120g potassium carbonate, 6.0mL dichloroethane (DCE), heat 85 ℃, react for 6 hours until the imidazopyridine reaction is complete (TLC monitoring by thin layer chromatography); pour the reaction mixture into 20 mL of water, extract with ethyl acetate (10 mL×5), combine the organic phases, and dry with anhydrous sodium sulfate; After removing the solvent, the residue was separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=6 / 1) to obtain 0.2981 g of the selenization product at the C3 position of the imidazopyridine derivative as a white solid, with a yield of 81%.
[0042] The specific response is shown in the following formula:
[0043]
[0044] The 1H NMR spectrum of the selenization product at the C3 position of the imidazopyridine derivative is as follows image 3 As shown, the spectrogram analysis data is as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com