Method for preparing ammonia gas based on radical chain reaction
A chemical chain and reaction technology, applied in chemical instruments and methods, inorganic chemistry, ammonia preparation/separation, etc., can solve the problems of low preparation efficiency, complex preparation process, high energy consumption, and achieve fast reaction speed, high concentration, high energy consumption, etc. The effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
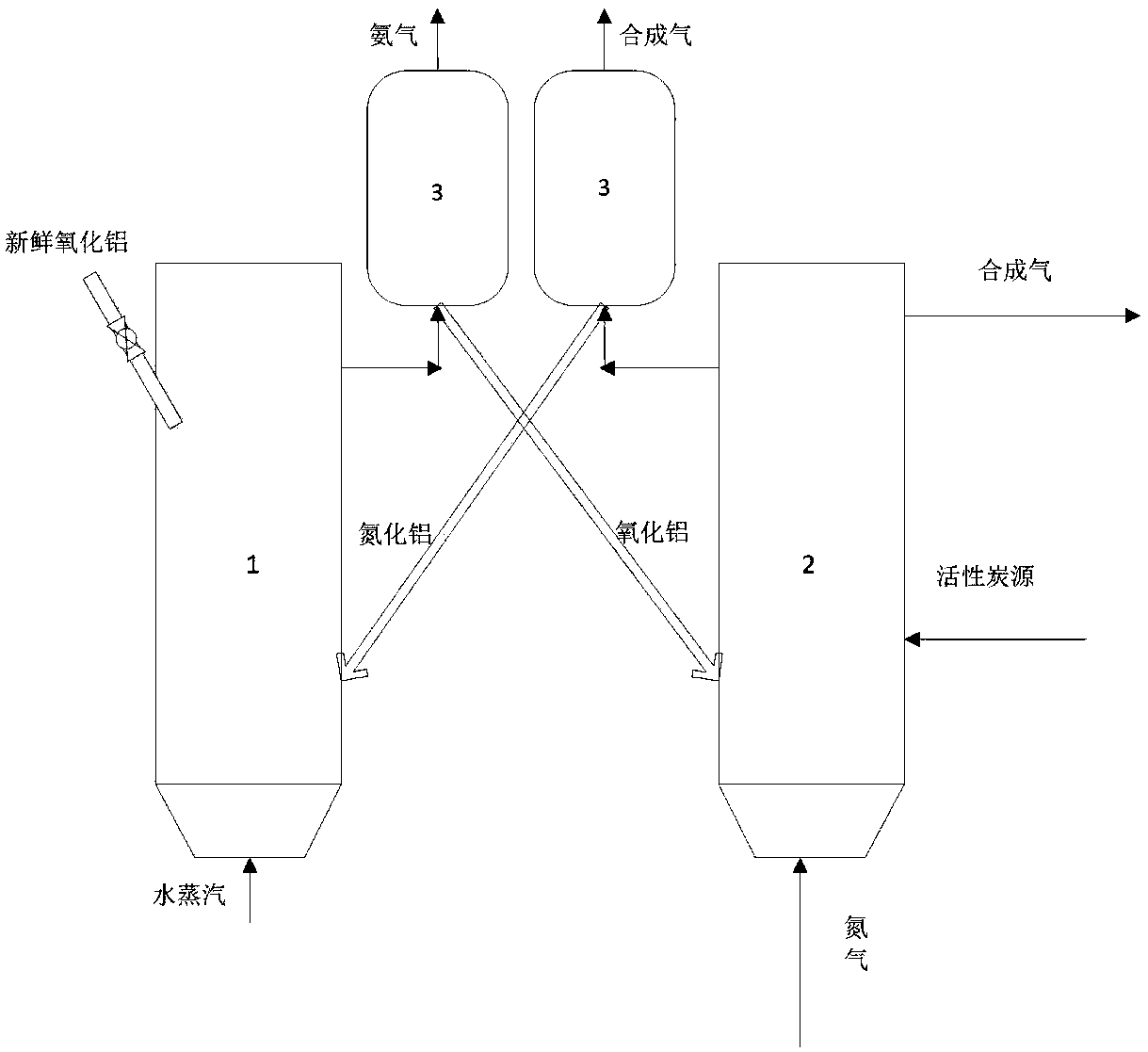
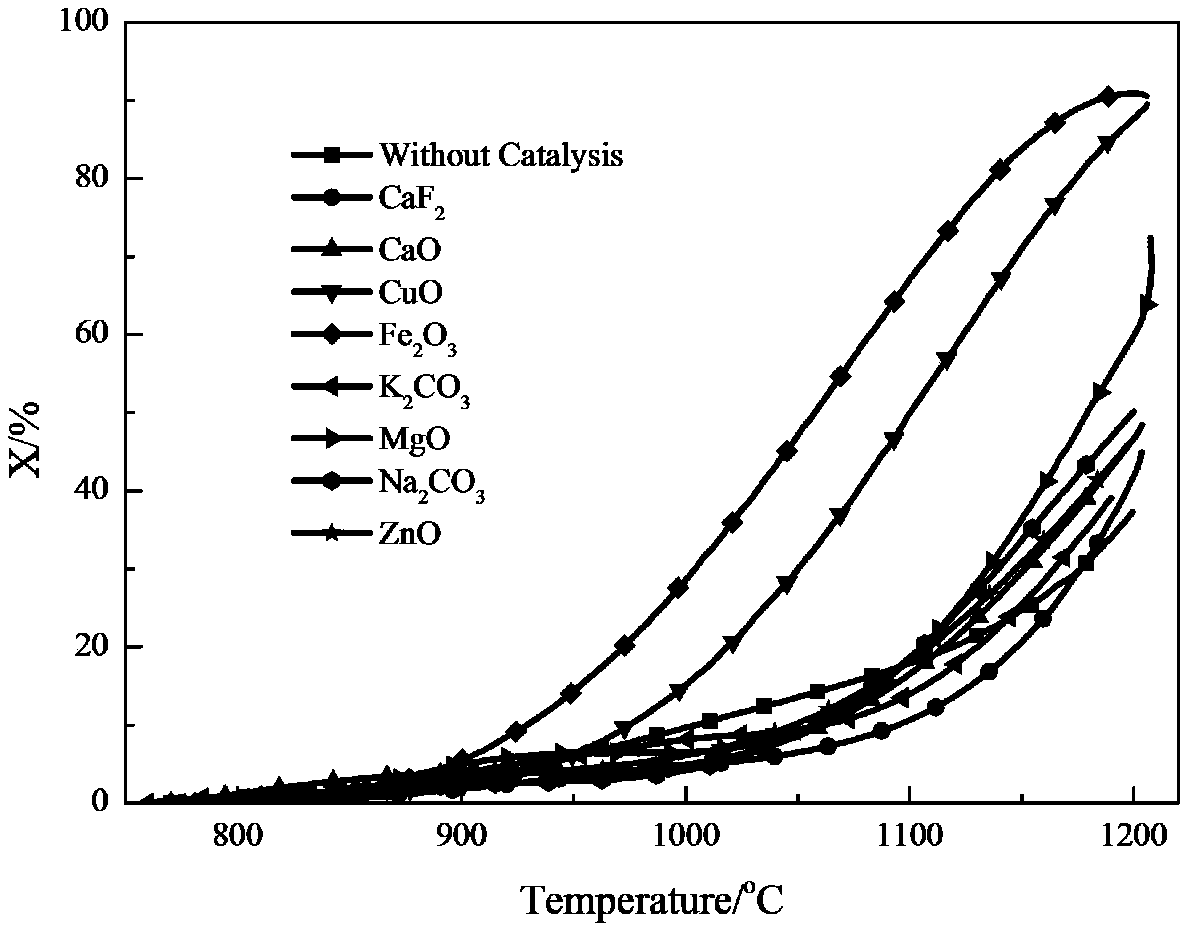
[0033] Coal, alumina, and calcium fluoride are continuously fed into the nitrogen absorption reactor 2 at a ratio of molar mass flow rate of 3:1:0.2, the temperature is maintained at 1400°C, the operation mode is a bubbling bed, and the fluidizing gas is nitrogen. After the carbon thermal reduction reaction of alumina, the chemical energy of coal char is transferred to aluminum nitride, and then the aluminum nitride is transported to the nitrogen release reactor 1. The temperature of the reactor is raised to 1200 ° C, and the operation mode adopts bubbling bed , the fluidization gas is water vapor, and the chemical energy of aluminum nitride is converted into ammonia gas after the nitrogen release reaction to generate high-concentration ammonia gas. The conversion rate of aluminum nitride during the nitrogen release process can reach 37.27%, such as figure 2 shown.
Embodiment 2
[0035] Coal, alumina, and calcium fluoride are continuously fed into the nitrogen absorption reactor 2 at a ratio of molar mass flow rate of 3:1:0.2, the temperature is maintained at 1400°C, the operation mode is a bubbling bed, and the fluidizing gas is nitrogen. After the carbon thermal reduction reaction of alumina, the chemical energy of coal char is transferred to aluminum nitride, and then the aluminum nitride is transported to the nitrogen release reactor 1 to be mixed with magnesium oxide, wherein the mass ratio of magnesium oxide to aluminum nitride is 5 : 95, the temperature of the reactor rises to 1200°C, the operation mode adopts bubbling bed, the fluidization gas is water vapor, and the chemical energy of aluminum nitride is converted into ammonia gas after the nitrogen release reaction to generate high-concentration ammonia gas, which releases The conversion rate of aluminum nitride in the nitrogen process can reach 72.42%, such as figure 2 shown.
Embodiment 3
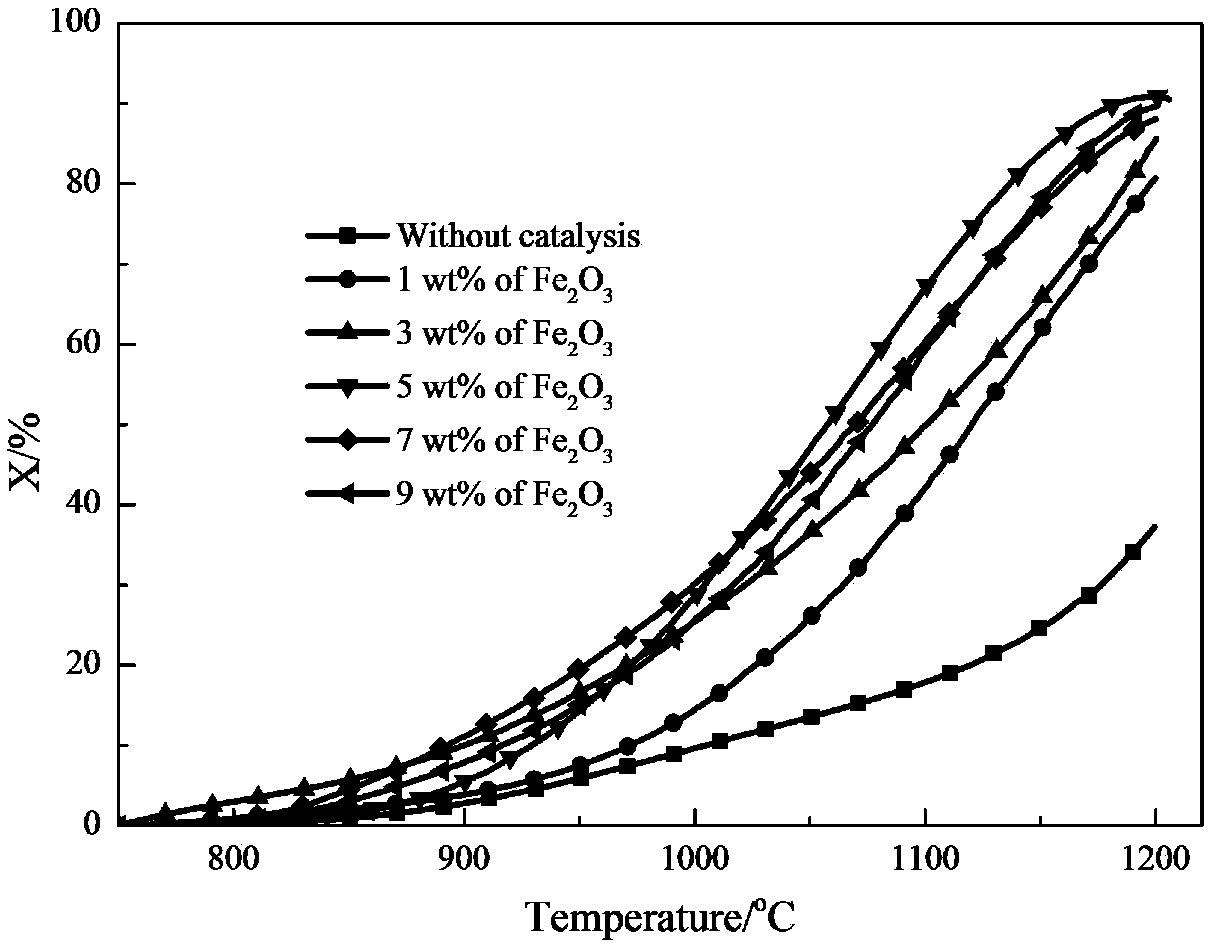
[0037] Coal, alumina, and calcium fluoride are continuously fed into the nitrogen absorption reactor 2 at a ratio of molar mass flow rate of 3:1:0.2, the temperature is maintained at 1400°C, the operation mode is a bubbling bed, and the fluidizing gas is nitrogen. After the carbon thermal reduction reaction of alumina, the chemical energy of coal char is transferred to aluminum nitride, and then the aluminum nitride is transported to the nitrogen release reactor 1 to be mixed with iron oxide, wherein the mass ratio of iron oxide to aluminum nitride is 5 : 95, the temperature of the reactor rises to 1200°C, the operation mode adopts bubbling bed, the fluidization gas is water vapor, and the chemical energy of aluminum nitride is converted into ammonia gas after the nitrogen release reaction to generate high-concentration ammonia gas, which releases The conversion rate of aluminum nitride in the nitrogen process can reach 90.51%, such as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com