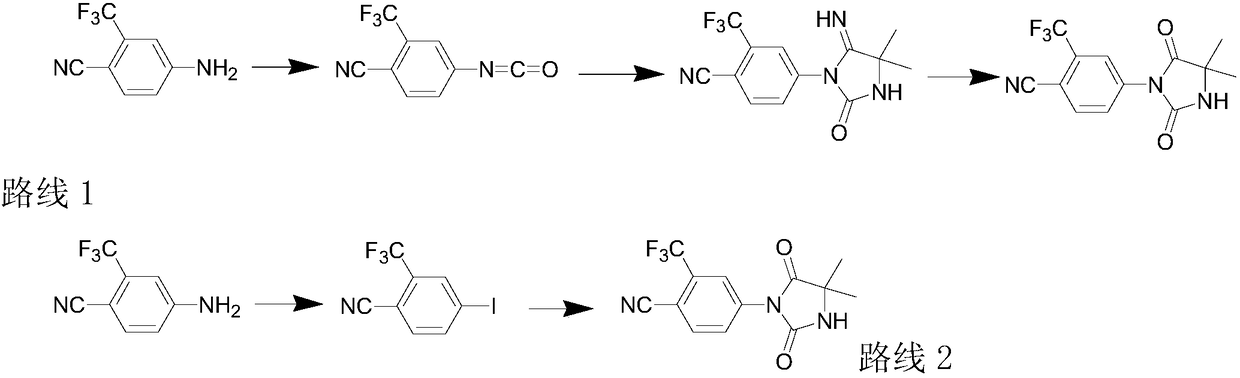
Preparation method of nilutamide
A cyanochlorobenzene and recrystallization technology, applied in the direction of organic chemistry, etc., can solve the problems of large amount of post-treatment wastewater, difficulty in purchasing propane, and high reaction temperature, and achieve the effects of low cost, less solvent in the system, and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 100mL dimethyl sulfoxide to a 250ml three-necked bottle, add 30g of 5,5-dimethylhydantoin at one time, then add 50g of 3-trifluoromethyl-4-cyanochlorobenzene, and then add 0.5g of Potassium iodide and 20 g of potassium hydroxide were heated to 80° C. for 6 hours. After the reaction was complete, cooled to room temperature, the reaction solution was poured into water, and 58 g of a yellow solid was obtained by filtration. The crude product was recrystallized from ethanol to obtain 48 g of pure nilutamide, with a yield of 66.3%.
Embodiment 2
[0021] Add 10L dimethyl sulfoxide to a 30L reactor, add 3Kg 5,5-dimethylhydantoin at one time, then add 5Kg3-trifluoromethyl-4-cyanochlorobenzene, and then add 50g of potassium iodide, 2Kg Potassium hydroxide, then heated to 80 ° C for 6 hours, after the reaction was complete, cooled to room temperature, the reaction solution was poured into water, and filtered to obtain 5.5 Kg of a yellow solid. The crude product was recrystallized from toluene to obtain 4.7 kg of pure nilutamide with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com