Preparation method of lenalidomide
A technology of lenalidomide and reaction temperature, applied in the field of preparation of lenalidomide, can solve the problems of high price of L-glutamine, unsuitable for industrial production, difficult purification and the like, and achieves good practicability and application value, The effect of high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of preparation method of lenalidomide, concrete steps are as follows:
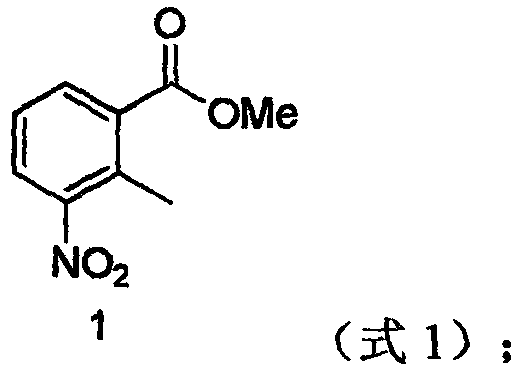
[0037] (1) Preparation of 2-methyl-3-nitrobenzoic acid methyl ester:
[0038] Add 1.81g (0.01mol) of 2-methyl-3-nitrobenzoic acid into 10ml of anhydrous methanol, add 0.003mol of triphosgene solid, add 3 drops of DMF, reflux for 5h, monitor by TLC, and recover by distillation under reduced pressure after the reaction Methanol was dissolved in ethyl acetate, washed with water, washed with saturated brine, distilled under reduced pressure, and dried to obtain 1.87 g of the product, with a yield of 95.8%;
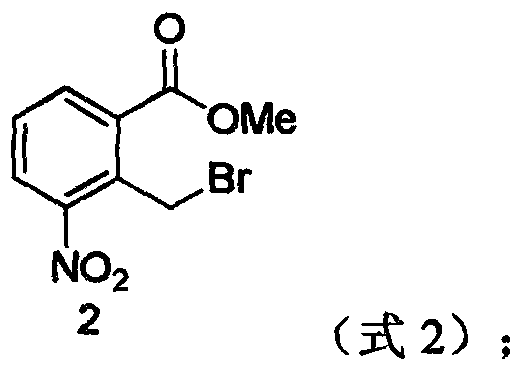
[0039] (2) Preparation of 2-bromomethyl-3-nitrobenzoic acid methyl ester:
[0040] Add 1.95g (0.01mol) of methyl 2-methyl-3-nitrobenzoate to 10ml of dichloroethane, add 3.54g (0.02mol) of NBS and heat to reflux, add a small amount of BPO, reflux for 8h, add 20ml of water, and separate the liquids , washing the organic phase with water, washing with saturated brine, distillation under reduce...
Embodiment 2
[0050] A kind of preparation method of lenalidomide, concrete steps are as follows:
[0051] (1) Preparation of 2-methyl-3-nitrobenzoic acid methyl ester:
[0052] Add 1.81g (0.01mol) of 2-methyl-3-nitrobenzoic acid into 10ml of anhydrous methanol, add 0.004mol of triphosgene solid, add 5 drops of DMF, reflux for 6h, monitor by TLC, and recover by distillation under reduced pressure after the reaction Methanol was dissolved in ethyl acetate, washed with water, washed with saturated brine, distilled under reduced pressure, and dried to obtain 1.88 g of the product, with a yield of 96.41%;
[0053](2) Preparation of 2-bromomethyl-3-nitrobenzoic acid methyl ester:
[0054] Add 1.95g (0.01mol) of methyl 2-methyl-3-nitrobenzoate to 10ml of chloroform, add 3.54g (0.02mol) of NBS, heat to reflux, add a small amount of BPO, reflux for 12h, add 20ml of water, separate liquid, organic phase Washing with water, washing with saturated brine, distillation under reduced pressure, and recr...
Embodiment 3
[0064] A kind of preparation method of lenalidomide, concrete steps are as follows:
[0065] (1) Preparation of 2-methyl-3-nitrobenzoic acid methyl ester:
[0066] Add 1.81g (0.01mol) of 2-methyl-3-nitrobenzoic acid into 10ml of absolute ethanol, add 0.01mol of thionyl chloride, add 3 drops of DMF, reflux for 5h, monitor by TLC, and distill under reduced pressure after the reaction is completed Methanol was recovered, dissolved in ethyl acetate, washed with water, washed with saturated brine, distilled under reduced pressure, and dried to obtain 1.64 g of the product with a yield of 84.10%;
[0067] (2) Preparation of 2-bromomethyl-3-nitrobenzoic acid methyl ester:
[0068] Add 1.95g (0.01mol) of methyl 2-methyl-3-nitrobenzoate to 10ml of carbon tetrachloride, add 3.54g (0.02mol) of NBS and heat to reflux, add a small amount of BPO, reflux at 60°C for 8h, add 20ml of water, Separation, washing the organic phase with water, washing with saturated brine, distillation under red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com