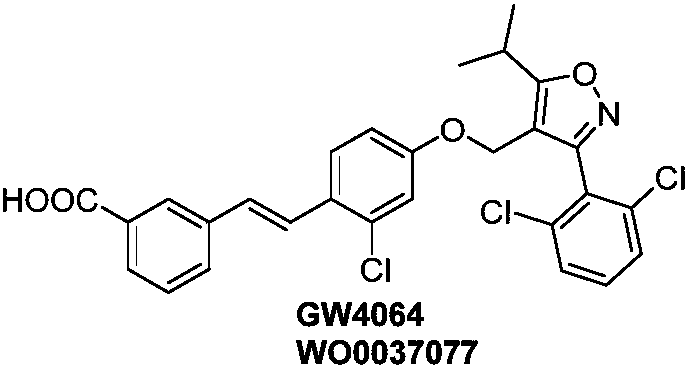
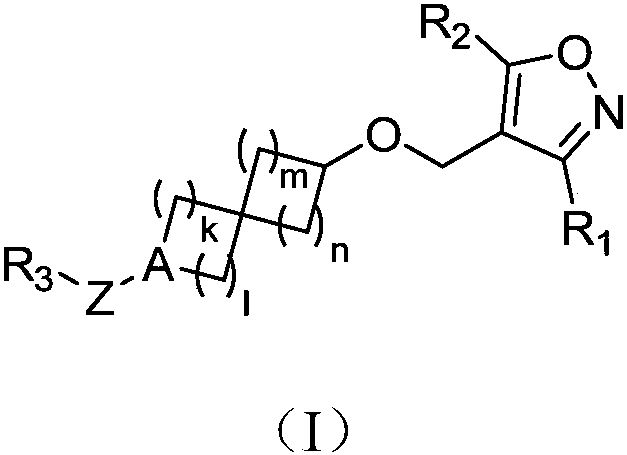
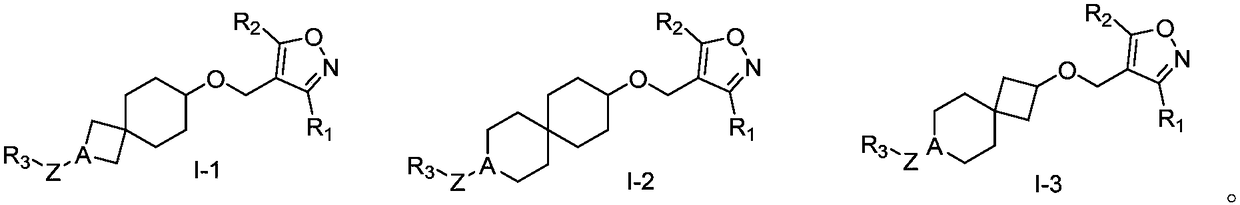
Spiro compound as well as preparation method, composition and application thereof
A technology of spiro compounds and oxides, applied in the field of spiro compounds, can solve problems such as itching and increase in low-density lipoprotein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1. Preparation of 4-(7-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-position)methoxy)-2-azaspiro[3.5 ]nonyl-2-position)benzoic acid (01)
[0126]
[0127] Intermediate 1-2:
[0128]
[0129] Dissolve ketone (0.990g, 0.0041mol, 1eq) in 10mL of methanol, add sodium borohydride (0.472g, 0.0124mol, 3eq) in batches under ice bath, and react for 30min under ice bath after addition, TLC shows that the conversion of raw materials is complete, concentrate It was dried, quenched with water and dilute hydrochloric acid, extracted three times with ethyl acetate, the organic layer was combined and washed with water, washed with saturated sodium bicarbonate solution, washed with brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 0.980 g of off-white solid.
[0130] Intermediates 1-3:
[0131]
[0132] Add 1-2 (0.980g, 0.0041mol, 1.5eq) to 10mL of anhydrous tetrahydrofuran, add 18-crown-6 (1.8g, 0.0069mol, 1.7eq) and potassium ter...
Embodiment 2
[0162] Example 2. Preparation of 3-(7-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-position)methoxy)-2-azaspiro[3.5 ]nonyl-2-position)benzoic acid (02)
[0163]
[0164] Compound 02 was prepared by referring to the steps described in Example 1 (Compound 01), and only the reaction raw materials were replaced accordingly to obtain the target compound. 1 H NMR (400MHz, CDCl 3 )δ: 7.42-7.29 (m, 5H), 7.11 (s, 1H), 6.65-6.62 (m, 1H), 4.30 (s, 2H), 3.55 (s, 2H), 3.53 (s, 2H), 3.25 -3.23(m, 1H), 2.16-2.12(m, 1H), 1.80-1.30(m, 8H), 1.15-1.10(m, 4H).ESI-MS m / z 525.8(M-H) - .
Embodiment 3
[0165] Example 3. Preparation of 6-(7-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-position)methoxy)-2-azaspiro[3.5 ]Nonyl-2-Nicotinic acid (03)
[0166]
[0167] Intermediate 3-2
[0168]
[0169] Add intermediate 1-1 (0.150g, 0.368mmol, 1eq), methyl 2-bromonicotinate (0.111g, 0.516mmol, 1.4eq), cuprous iodide (0.029g, 0.147mmol , 0.4eq), L-proline (0.018g, 0.147mmol, 0.4eq) and 5ml DMSO, heated to 120 degrees and stirred overnight, TLC showed that the conversion of raw materials was complete, quenched with water, extracted three times with ethyl acetate, and the organic layer Combined, washed with water and brine in turn, dried and concentrated to dryness, silica gel column chromatography (PE / EA=10 / 1, 5 / 1) gave 140 mg of light yellow oil.
[0170] Compound 03
[0171]
[0172] Intermediate 3-2 (0.140mg, 0.258mmol, 1.0eq) was dissolved in 5ml THF and 5ml MeOH, potassium hydroxide (0.051mg, 0.774mmol, 3.0eq) was added, and 1ml of water was heated to 60°C and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com