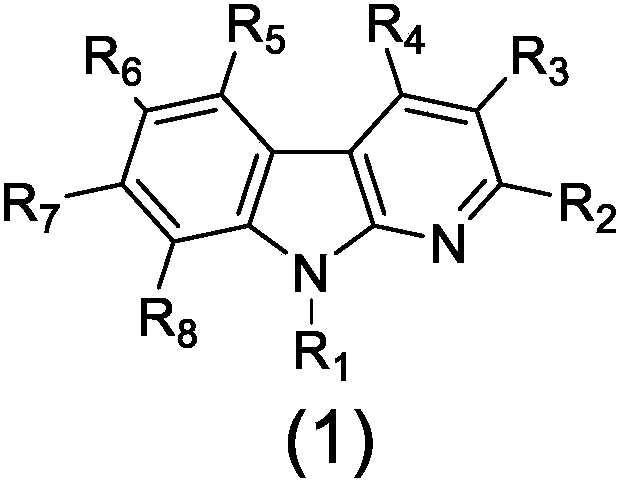
Application of alpha-carboline derivative in preparation of anti-myocardial anoxia reoxygenation injury drug
A technology of derivatives and compounds, applied in the field of drug synthesis, can solve the problems of weak specificity, obvious tolerance of long-term use, low curative effect, etc., and achieve a good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
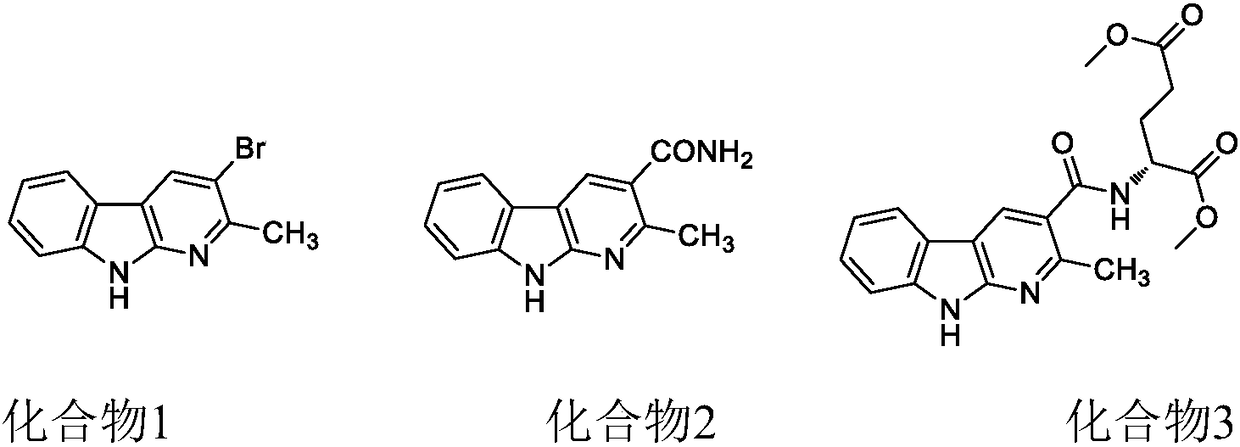
[0043] Embodiment 1 compound 1---the preparation of 2-methyl 3-bromo-alpha carbolin
[0044] Step 1: Preparation of 1-(5-bromo-6-methyl-2-pyridyl)-1H-benzotriazole
[0045] At 120°C, 2,5-dibromo-6-picoline (3.0g, 11.96mmol) and benzotriazole (2.85g, 23.91mmol) were mixed and dissolved in DMSO (100mL), and K 2 CO 3(11.26g, 81.47mmol), reacted under stirring and reflux for 48h, left to cool, added 300mL water to dissolve, extracted with EtOAc, then backwashed with water, concentrated under reduced pressure, the residue was purified by silica gel column chromatography to obtain the product, the yield 39.2%.
[0046] Step 2: Preparation of 2-methyl 3-bromo-αcarbolin
[0047] At 160°C, take polyphosphoric acid (80mL) in a reaction flask, add 1-(5-bromo-6-methyl-2-pyridyl)-1H-benzotriazole (2.03g, 7.0mmol ) in polyphosphoric acid, stirred for 4 hours, added 150mL of water to dissolve, and used Na 2 CO 3 Adjust pH=9, filter, and extract the filtrate with EtOAc, anhydrous Na 2 ...
Embodiment 2
[0048] Embodiment 2 compound 2---the preparation of 2-methyl 3-amido-alpha carbolin
[0049] Step 1: Preparation of 1-(5-cyano-6-methyl-2-pyridyl)-1H-benzotriazole
[0050] At 120°C, take 2-methyl 3-bromo-αcarbolin (compound 1, 1.0g, 3.46mmol), potassium ferrocyanide (382.19mg, 1.04mmol), bis(triphenylphosphine) di Palladium chloride (121.38mg, 172.93mmol), sodium carbonate (1.1g, 10.38mmol) and palladium acetate (77.65mg, 345.86mmol) in a reaction flask, and measure N,N-dimethylacetamide (18ml) Placed in a reaction flask, under the protection of nitrogen, the reaction was stirred for 4-8h. After completion of the reaction, add 100mL of water to dissolve, filter, and extract the filtrate with EtOAc, anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, the residue was purified by silica gel column chromatography to obtain 1-(5-cyano-6-methyl-2-pyridyl)-1H-benzotriazole with a yield of 94%.
[0051] Step 2: Preparation of 2-methyl 3-amido-αcarbolin
[...
Embodiment 3
[0053] Embodiment 3 compound 3---the preparation of 2-methyl 3-glutamic acid diester base-alpha carbolin
[0054] Step 1: Preparation of 2-methyl 3-carboxy-αcarbolin
[0055] At 120°C, take 2-methyl 3-amido-αcarbolin (compound 2, 1.0g, 4.42mmol) in a reaction flask, add 20ml of concentrated hydrochloric acid, stir the reaction for 24h, and use K 2 CO 3 Adjust pH=9, filter, and extract the filtrate with EtOAc, anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and purify the residue by silica gel column chromatography to obtain 2-methyl 3-carboxy-αcarbolin with a yield of 97.2%.
[0056] Step 2: Preparation of 2-methyl-3-glutamate dimethyl-alpha carboline
[0057] Take 2-methyl 3-carboxy-αcarbolin (500.00mg, 2.21mmol), dimethyl glutamate (464.61mg, 2.65mmol), Carter condensing agent (1.47g, 3.32mmol), triethanolamine (5ml ) in a reaction flask, stirred at room temperature for 20 h. Add 120mL water to dissolve, filter, and extract the filtrate with EtOAc, anhydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com