Environmentally-friendly synthesis method of bisphenol fluorene
A technology of green synthesis and bisphenol fluorene, which is applied in the field of catalysis, can solve the problems of harsh reaction conditions, expensive catalysts, and low process requirements, and achieve the effects of mild reaction conditions, excellent catalytic effect, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) NiFe 2 o 4 preparation of
[0031] 3.0 mmol FeCl 3 ·6H 2 O and 1.5 mmol NiSO 4 ·6H 2 O was dissolved in 40.0 mL of ethylene glycol solution, and then 22.0 mmol of sodium acetate and 6.0 mmol of CTAB were added to it and ultrasonically treated for 30 min to obtain a mixture; then reacted in a water bath at 180°C for 24 h to obtain a suspension, and the suspension was naturally After cooling to room temperature, centrifuge, wash and dry at 105°C for 12 hours to obtain NiFe 2 o 4 magnetic nanocrystals;
[0032] 2) NiFe 2 o 4 Surface TiO 2 clad
[0033] Same as Example 1
[0034] 3) Loading of ionic liquids
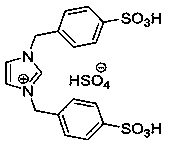
[0035] 1g of NiFe 2 o 4 @TiO 2 The carrier was placed in 20mL aqueous solution, and then ionic liquid W was added to disperse for 2h under ultrasonic conditions of 150w, and then it was placed in a vacuum oven at 70°C for 12h to obtain an immobilized ionic liquid catalyst; wherein, ionic liquid W and NiFe 2 o 4 @TiO 2 The mass ratio of carrier is ...
Embodiment 2
[0039] 1) NiFe 2 o 4 preparation of
[0040] 3.0 mmol FeCl 3 ·6H 2 O and 1.5 mmol NiSO 4 ·6H 2 O was dissolved in 40.0 mL of ethylene glycol solution, and then 22.0 mmol of sodium acetate and 6.0 mmol of CTAB were added to it and ultrasonically treated for 30 minutes to obtain a mixture; then reacted in a water bath at 220°C for 12 hours to obtain a suspension, and the suspension was naturally After cooling to room temperature, centrifuge, wash and dry at 105°C for 12 hours to obtain NiFe 2 o 4 magnetic nanocrystals;
[0041] 2) NiFe 2 o 4 Surface TiO 2 clad
[0042] Same as Example 1
[0043] 3) Loading of ionic liquids
[0044] 1g of NiFe 2 o 4 @TiO 2 The carrier was placed in 20mL aqueous solution, and then ionic liquid W was added to disperse for 30min under 300w ultrasonic conditions, and then it was placed in a vacuum oven at 70°C for 12h to obtain an immobilized ionic liquid catalyst; wherein, ionic liquid W and NiFe 2 o 4 @TiO 2 The mass ratio of car...
Embodiment 3
[0048] 1) NiFe 2 o 4 preparation of
[0049] 3.0 mmol FeCl 3 ·6H 2 O and 1.5 mmol NiSO 4 ·6H 2 O was dissolved in 40.0 mL of ethylene glycol solution, and then 22.0 mmol of sodium acetate and 6.0 mmol of CTAB were added to it and ultrasonically treated for 30 min to obtain a mixture; then reacted in a water bath at 200°C for 18 h to obtain a suspension, and the suspension was naturally After cooling to room temperature, centrifuge, wash and dry at 105°C for 12 hours to obtain NiFe 2 o 4 magnetic nanocrystals;
[0050] 2) NiFe 2 o 4 Surface TiO 2 clad
[0051] Same as Example 1
[0052] 3) Loading of ionic liquids
[0053] 1g of NiFe 2 o 4 @TiO 2 The carrier was placed in 20mL aqueous solution, then ionic liquid W was added to disperse for 1h under ultrasonic conditions of 250w, and then it was placed in a vacuum oven at 70°C for 12h to obtain an immobilized ionic liquid catalyst; wherein, ionic liquid W and NiFe 2 o 4 @TiO 2 The mass ratio of carrier is 25wt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com