Drug composition containing vardenafil hydrochloride and orally disintegrating tablet containing drug composition as well as preparation of orally disintegrating tablet and application of drug composition
A technology of vardenafil hydrochloride and vardenafil, which is applied in the direction of drug combination, medical preparation of non-active ingredients, drug delivery, etc. It can solve the problem of long disintegration time of orally disintegrating tablets, poor uniformity of drug content, etc. problem, to achieve good drug content uniformity and reduce energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
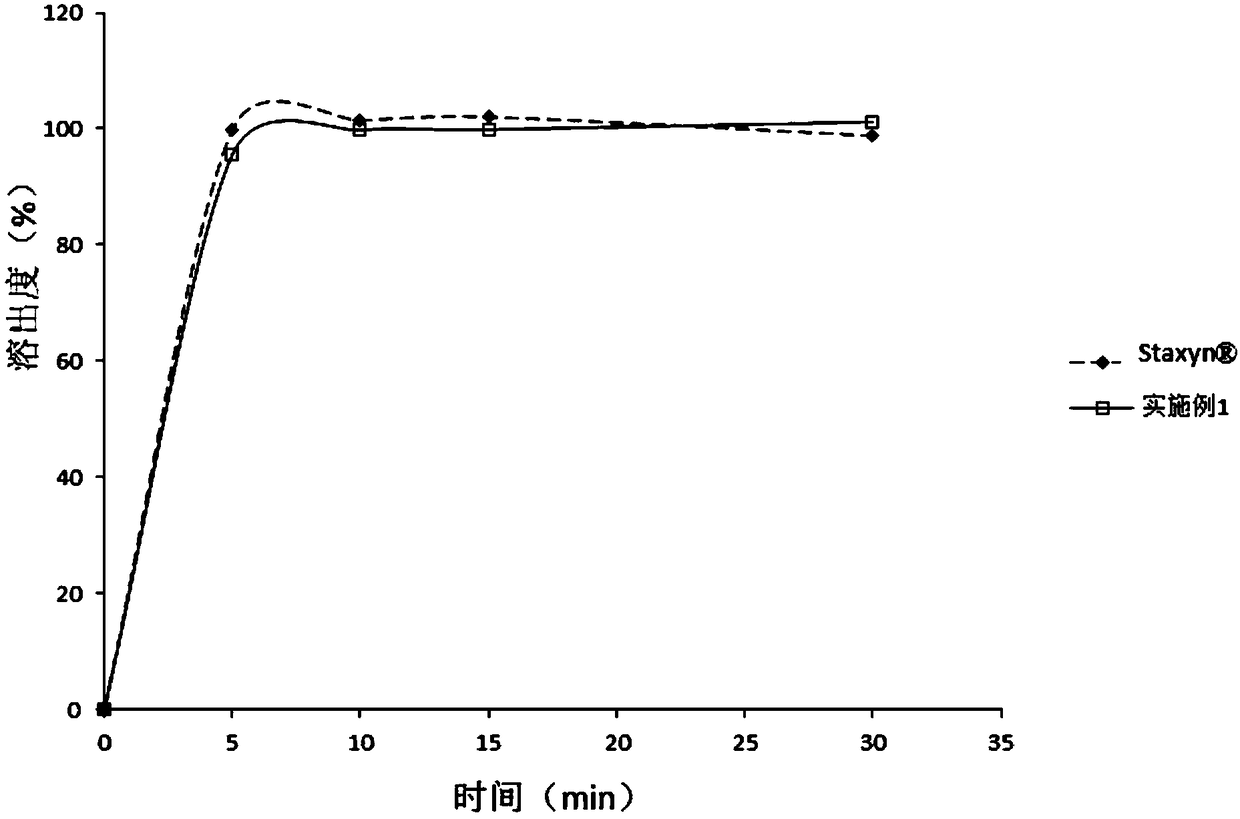
Embodiment 1
[0080]
[0081]
[0082] Preparation:
[0083] Pass vardenafil hydrochloride trihydrate through a 80-mesh sieve, mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, fructose, essence, and sucralose all pass through a 60-mesh sieve, magnesium stearate, colloidal Silica is passed through a 30-mesh sieve for subsequent use. Take microcrystalline cellulose, vardenafil hydrochloride trihydrate (its D 90 The particle size is 45 μm), sucralose, fructose, essence, placed in a V-type mixer or a universal mixer for pre-mixing for 5 minutes. Then add the prescribed amount of low-substituted hydroxypropyl cellulose and mannitol in turn and mix for 15 minutes. Put the material in the hopper of a dry granulator and granulate, transfer the prepared granules to a mixer, add the prescribed amount of colloidal silicon dioxide and magnesium stearate and mix for 3 minutes. After mixing evenly, press into tablets, and the prepared tablets are not coated. Among ...
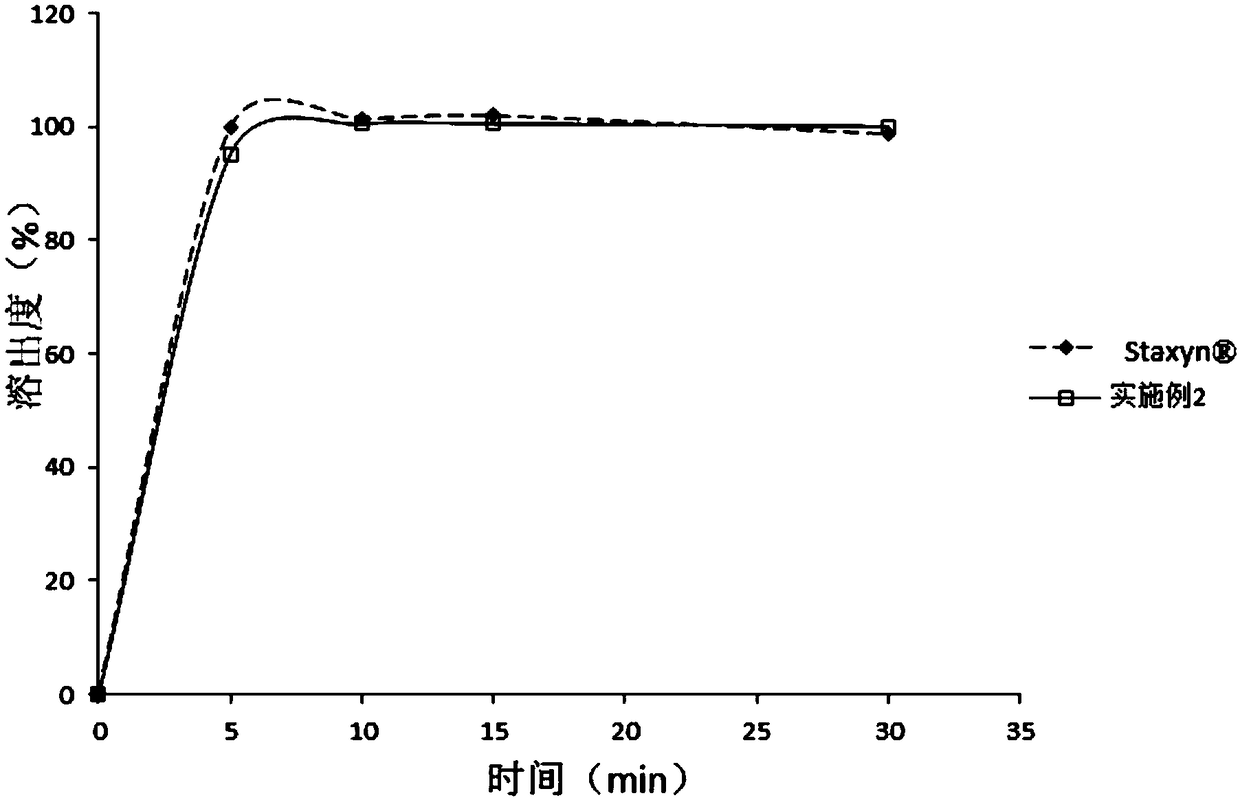
Embodiment 2
[0085]
[0086] Preparation:
[0087] Pass vardenafil hydrochloride through a 80-mesh sieve, microcrystalline cellulose, sorbitol, xylitol, crospovidone, aspartame, and flavors through a 60-mesh sieve, and magnesium lauryl sulfate through a 30-mesh sieve Sieve and set aside. Weigh 1 / 2 microcrystalline cellulose, xylitol, vardenafil hydrochloride (its D 90 The particle size is 105 μm), aspartame, essence, and cross-linked povidone, and pre-mixed in a V-type mixer or a universal mixer for 5 minutes. Then add the remaining 1 / 2 of the prescription amount of microcrystalline cellulose and sorbitol and mix for 15 minutes. Add the prescribed amount of magnesium lauryl sulfate and mix for 3 minutes. After mixing evenly, it is directly pressed into tablets, and the prepared tablets are not coated. Wherein, the tabletting parameters are consistent with that of Example 1, with a shallow concave circular punch. Test this tablet according to described test method, the results are s...
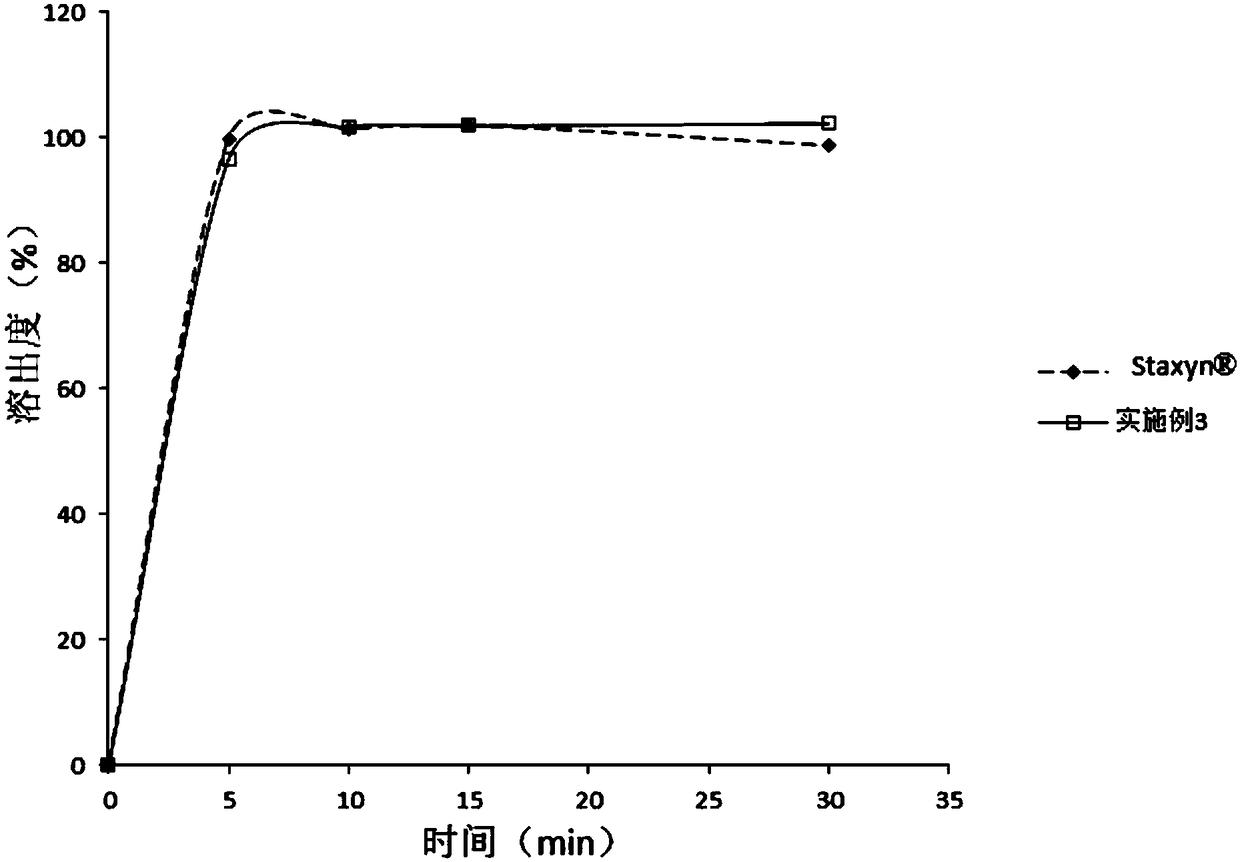
Embodiment 3
[0089]
[0090] Preparation:
[0091] Pass vardenafil hydrochloride trihydrate through a 80-mesh sieve, microcrystalline cellulose, anhydrous calcium hydrogen phosphate, crospovidone, sorbitol, xylitol, and essence all pass through a 60-mesh sieve, and magnesium stearate pass through a 60-mesh sieve. 30 mesh sieve for use. Sorbitol, vardenafil hydrochloride trihydrate (its D 90 The particle size is 45 μm), xylitol, essence, crospovidone, anhydrous calcium hydrogen phosphate, pre-mixed in a V-type mixer or a universal mixer for 5 minutes. Then add the prescribed amount of microcrystalline cellulose and mix for 15 minutes. Put the material in the dry granulator hopper for granulation, transfer the obtained granules to the mixer, add the prescribed amount of magnesium stearate and mix for 3 minutes. After mixing evenly, press into tablets, and the prepared tablets are not coated. Among them, the parameters of dry granulation and tabletting are consistent with those of Exam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com