Pharmaceutical composition for treating colon cancer and application thereof
A composition and colon cancer technology, applied in the field of medicine, can solve problems such as unsatisfactory therapeutic effects, side effects, and hidden dangers of drug safety of Chinese patent medicines, and achieve the goals of improving drug safety, inhibiting proliferation, and reducing the risk of bleeding Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Compound Tablets
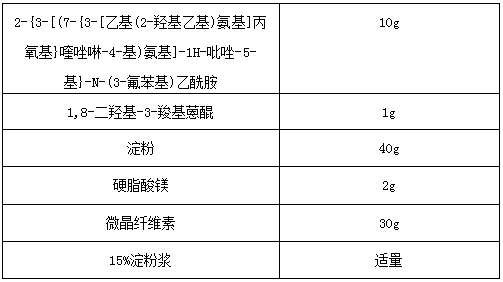
[0025]
[0026] Wherein, the dosage of 15% starch slurry in the tablet is not strictly limited, and those skilled in the art can make a suitable selection and determination according to the actual situation. Give a detailed description.
[0027] Preparation process: 2-{3-[(7-{3-[Ethyl(2-hydroxyethyl)amino]propoxy}quinazolin-4-yl)amino]-1H-pyrazole-5- Base}-N-(3-fluorophenyl)acetamide, 1,8-dihydroxy-3-carboxyanthraquinone, microcrystalline cellulose and starch are mixed evenly, and an appropriate amount of 15% starch slurry is added to make soft material, and then Granulate through a 16-mesh sieve, dry the wet granules at 60°C, granulate the dry granules through a 16-mesh sieve, sift out the fine powder in the dry granules, mix with magnesium stearate, then mix with the dry granules, and press into tablets , can obtain compound tablet of the present invention.
Embodiment 2
[0029] Preparation of Compound Dispersible Tablets
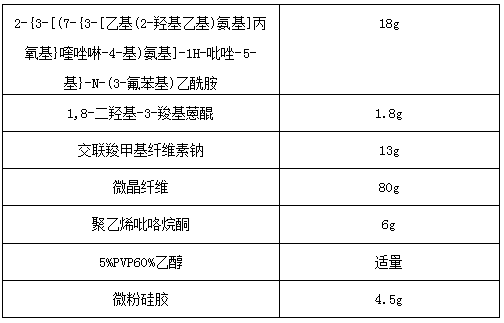
[0030]
[0031] Wherein, the dosage of 5%PVP60% ethanol in the tablet is not strictly limited, and those skilled in the art can make a suitable selection and determination according to the actual situation, as long as the dosage is convenient for the preparation of dispersible tablets. Give a detailed description.
[0032] Preparation process: Weigh 2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}quinazolin-4-yl)amino]-1H-pyrazole -5-yl}-N-(3-fluorophenyl)acetamide and 1,8-dihydroxy-3-carboxyanthraquinone, with microcrystalline fiber, croscarmellose sodium and polyvinylpyrrolidone as disintegration Debonding agent, 5%PVP60% ethanol is binding agent, micropowder silica gel is glidant, with fluidized bed one-step granulation, finally tabletting, can obtain compound dispersible tablet of the present invention.
Embodiment 3
[0034] Preparation of Compound Granules
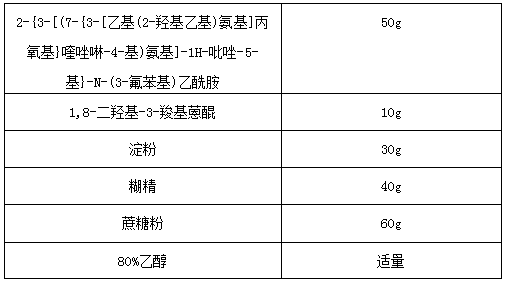
[0035]
[0036] Wherein, the dosage of 80% ethanol in the tablet is not strictly limited, and those skilled in the art can make a suitable selection and determination according to the actual situation, as long as the dosage is convenient for the preparation of granules, and will not be detailed here. describe.
[0037] Preparation process: Weigh 2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}quinazolin-4-yl)amino]-1H-pyrazole -5-yl}-N-(3-fluorophenyl)acetamide, 1,8-dihydroxy-3-carboxyanthraquinone, starch, dextrin and sucrose powder were mixed evenly, and an appropriate amount of 80% ethanol was added to In the mixed powder, mix evenly, make a soft material, pass through a 18-mesh nylon sieve to make wet granules, dry at about 60°C, granulate with a 20-mesh sieve, and pack separately to obtain the compound granules of the present invention.
[0038] Inhibitory effect of the pharmaceutical composition of the present invention on c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com