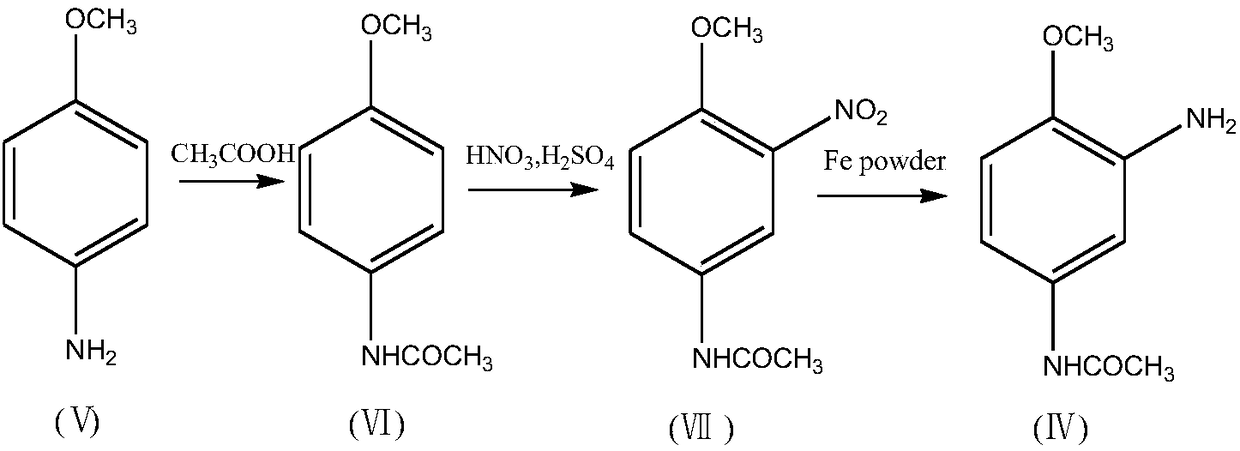
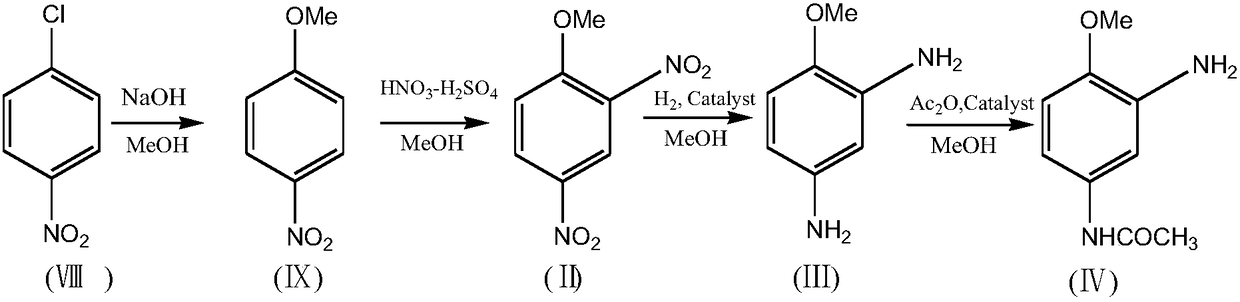
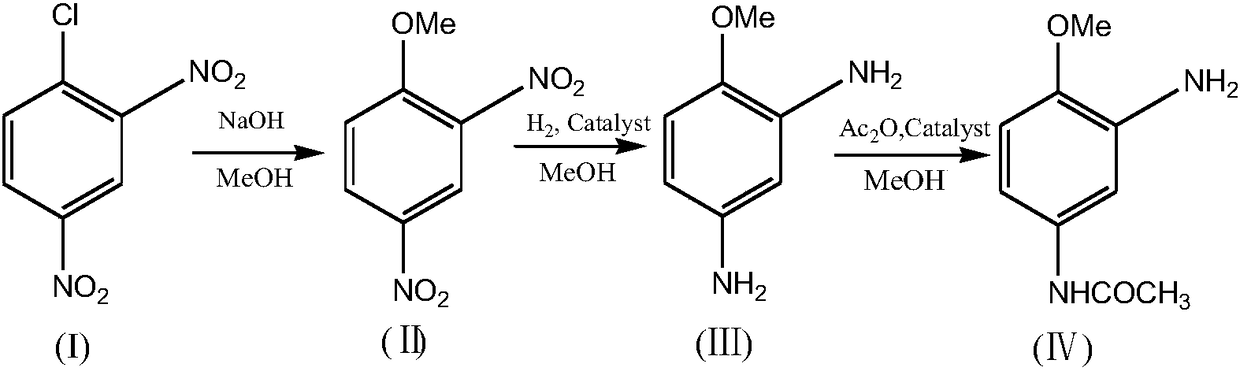
Process for synthesizing 2-amino-4-acetylaminoanisole
A technology of acetaminoanisole and diaminoanisole, which is applied in the field of preparation of organic compounds, can solve problems such as unstable yield and quality of target compounds, unsuitability for industrial production, and a large amount of waste solids and waste liquids. The effect of reducing the chance of by-product formation, reducing the dosage, and high industrial safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Weigh 100g of 2,4-dinitrochlorobenzene (I), 200g of methanol, and 2.5g of PEG2000 into a four-neck flask, heat up to 60°C, and stir; add 20g of sodium hydroxide in batches within 2.5 to 3.0 hours, Add sodium hydroxide, keep warm, and the reaction ends. Methanol was recovered by distillation and washed with water to obtain 95 g (99%) of 2,4-dinitroanisole (II), with a yield of 96.1%.
[0056] The NMR data of 2,4-dinitroanisole (II) are as follows: 1 HNMR (500MHz, CDCl3): δ8.82 (d, 1H), 8.4-8.5 (dd, 1H), 8.73 (d, 1H), 3.77 (s, 3H).
Embodiment 2
[0058] Put 95g (HPLC purity 99.2%) 2,4-dinitroanisole (II), 380g methanol and 0.95g Pd / C catalyst into a four-necked flask, add 85.5g monoformic acid hydrazine salt, react at room temperature, and react complete. The catalyst Pd / C was recovered by filtration. The HPLC purity of the active ingredient 2,4-diaminoanisole (III) in the mother liquor was 99.1%, and the conversion rate was 100%. The mother liquor was directly used in the acylation reaction without treatment.
[0059] Add 19.0g of sodium carbonate to the above mother liquor, cool to -5°C, add 47.2g of acetic anhydride, after the addition is complete, raise the temperature to 5°C and keep warm until the reaction is complete. Distill 304g of methanol, add water to the residue, cool and crystallize, and suction filter to obtain 75.1g (HPLC purity 99.5%) 2-amino-4-acetamidoanisole (IV), 2,4-diacetamidobenzene The amount of methyl ether generated is below 1% (measured by HPLC), and the total yield of the two steps is 89.0...
Embodiment 3
[0062] Weigh 100g of 2,4-dinitrochlorobenzene (I), 150g of methanol, and 1.6g of benzyltriethylammonium chloride into a four-neck flask, heat up to 70°C, and stir; within 2.5 to 3.0 hours, Add 19.7g of sodium hydroxide in batches, after adding the sodium hydroxide, keep warm, and the reaction ends. Methanol was recovered by distillation and washed with water to obtain 92.8 g (HPLC purity 99.1%) of 2,4-dinitroanisole (II), with a yield of 93.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com