Inactivated probiotic bacillus injection
A technology of Bacillus and Bacillus subtilis, applied in the field of antibacterial and antiviral injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Inoculate Bacillus subtilis (Latin name: Bacillus subtilis, purchased from China Industrial Microorganism Culture Collection Management Center, preservation number: CICC 10012) into the medium (weigh 10g peptone, 10g sodium chloride, 5g yeast powder, add water to 1000mL , adjusted the pH to 7.0, sterilized at 121°C for 20 minutes), cultured in a constant temperature shaker at 37°C at a speed of 140r / min for 18-24 hours, then centrifuged at 3000 rpm for 5 minutes, removed the supernatant culture solution and retained the precipitate, added sterile Wash the precipitate with normal saline, centrifuge for 5 minutes, repeat the washing 3 times, add sterile normal saline, mix with the precipitate, and make a suspension. Take a certain amount of Bacillus subtilis physiological saline suspension, and count the bacteria on a THOMA bacterial counting plate, so that each ml suspension contains 1×10 9 Bacillus subtilis bacteria. The prepared Bacillus subtilis physiological saline ...
Embodiment 2
[0022] Inoculate Bacillus licheniformis (Bacillus licheniformis, Latin name: Bacillus licheniformis, purchased from China Industrial Microorganism Culture Collection Management Center, preservation number: CICC24236) in the medium (weigh 10g peptone, 10g sodium chloride, 5g yeast powder, Add water to 1000mL, adjust the pH to 7.0, sterilize at 121°C for 20min to prepare), incubate in a constant temperature shaker at 37°C at a speed of 140r / min for 18-24 hours, then centrifuge at 3000rpm for 5 minutes, remove the upper culture solution and retain the precipitate, Add sterile normal saline to wash the precipitate, centrifuge for 5 minutes, repeat the washing 3 times, add sterile normal saline, mix with the precipitate, and make a suspension. Take a certain amount of Bacillus licheniformis saline suspension, and count the bacteria on the THOMA bacterial counting plate, so that each ml suspension contains 1×10 9 Bacillus licheniformis. The prepared Bacillus licheniformis saline su...
Embodiment 3
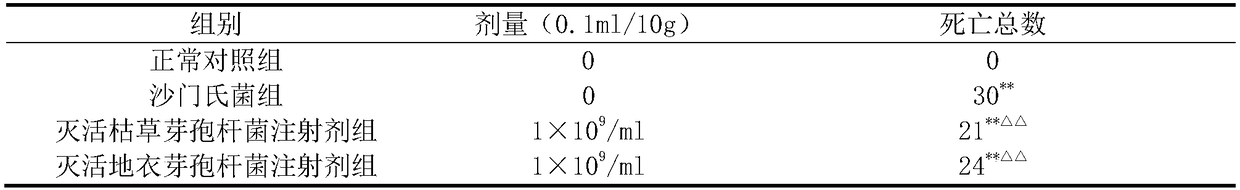
[0024] The prevention and control effects of the inactivated Bacillus subtilis injection prepared in Example 1 and the inactivated Bacillus licheniformis injection prepared in Example 2 on Salmonella-infected mice were tested. Divide clean-grade Kunming mice with a body weight of 18-22g into 3 groups, namely the normal control group, the Salmonella group, the inactivated Bacillus subtilis injection group and the inactivated Bacillus licheniformis injection group, with 30 mice in each group, male and female Half and half. The mice in the normal control group and the Salmonella group were injected with sterile normal saline in the tail vein, and the inactivated Bacillus subtilis injection group and the inactivated Bacillus licheniformis injection group were respectively injected with corresponding drugs with a volume of 0.1 mL / 10 g. 24 hours after administration once, the mice in Salmonella group, inactivated Bacillus subtilis injection group and inactivated Bacillus licheniform...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com