Purification method of 3-methyl-3-butene-1-ol
A purification method and technology of butene, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as solvent pyrolysis, difficult selection of solvents, and decrease in yield, so as to reduce operating temperature and enhance Miscibility, the effect of reducing self-aggregation loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
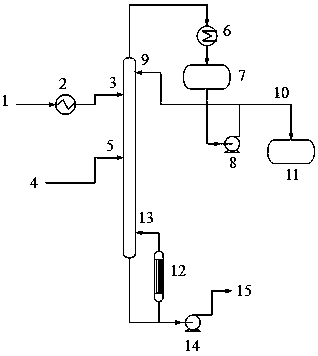
[0035] as attached figure 1 As shown, the top pressure of the extractive distillation tower is -0.075MPa, the total number of theoretical plates is 68, the solvent inlet is the 12th theoretical plate, and the raw material import is the 28th theoretical plate. The solvent is pure n-dodecane, the solvent flow rate is 2500kg / h (5 times the mass flow rate of the raw material), and the solvent feed temperature is 90°C. The operating reflux ratio was 8.
[0036] The flow rate of the top product obtained after stable operation is 378kg / h (containing 98.94% of 3-methyl-3-buten-1-ol, 0.98% of isoamyl alcohol, and 0.08% of solvent); the flow rate of the bottom product obtained is It is 2622kg / h (containing 0.04% of 3-methyl-3-buten-1-ol, 4.63% of isoamyl alcohol, and 95.34% of solvent).
[0037] The calculated yield of 3-methyl-3-buten-1-ol in the top product is 99.73%, and the yield of isoamyl alcohol in the bottom product is 97.04%.
Embodiment 2
[0039] as attached figure 1 As shown, the top pressure of the extractive distillation tower is -0.09MPa, the total number of theoretical plates is 50, the solvent inlet is the 8th theoretical plate, and the raw material inlet is the 25th theoretical plate. The solvent contains 10% n-octanol (the rest is n-dodecane), the solvent flow rate is 2500kg / h (5 times the mass flow rate of the raw material), and the solvent feed temperature is 80°C. The operating reflux ratio was 5.
[0040] The flow rate of the top product obtained after stable operation is 376.5kg / h (containing 99.29% of 3-methyl-3-buten-1-ol, 0.57% of isoamyl alcohol, and 0.14% of solvent); the obtained bottom product The flow rate is 2623.5kg / h (containing 0.04% of 3-methyl-3-buten-1-ol, 4.68% of isoamyl alcohol, and 95.27% of solvent).
[0041] The calculated yield of 3-methyl-3-buten-1-ol in the overhead product is 99.69%, and the yield of isoamyl alcohol in the bottom product is 98.28%.
Embodiment 3
[0043] as attached figure 1 As shown, the top pressure of the extractive distillation tower is -0.085MPa, the total number of theoretical plates is 75, the solvent inlet is the 14th theoretical plate, and the raw material import is the 45th theoretical plate. The solvent contains 15% n-octanol (the rest is n-dodecane), the solvent flow rate is 2000kg / h (4 times the mass flow rate of the raw material), and the solvent feed temperature is 85°C. The operating reflux ratio was 6.
[0044]The flow rate of the top product obtained after stable operation is 377kg / h (containing 99.37% of 3-methyl-3-buten-1-ol, 0.59% of isoamyl alcohol, and 0.04% of solvent); the flow rate of the obtained bottom product It is 2123kg / h (containing 0.02% of 3-methyl-3-buten-1-ol, 5.78% of isoamyl alcohol, and 94.20% of solvent).
[0045] The calculated yield of 3-methyl-3-buten-1-ol in the top product is 99.90%, and the yield of isoamyl alcohol in the bottom product is 98.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com