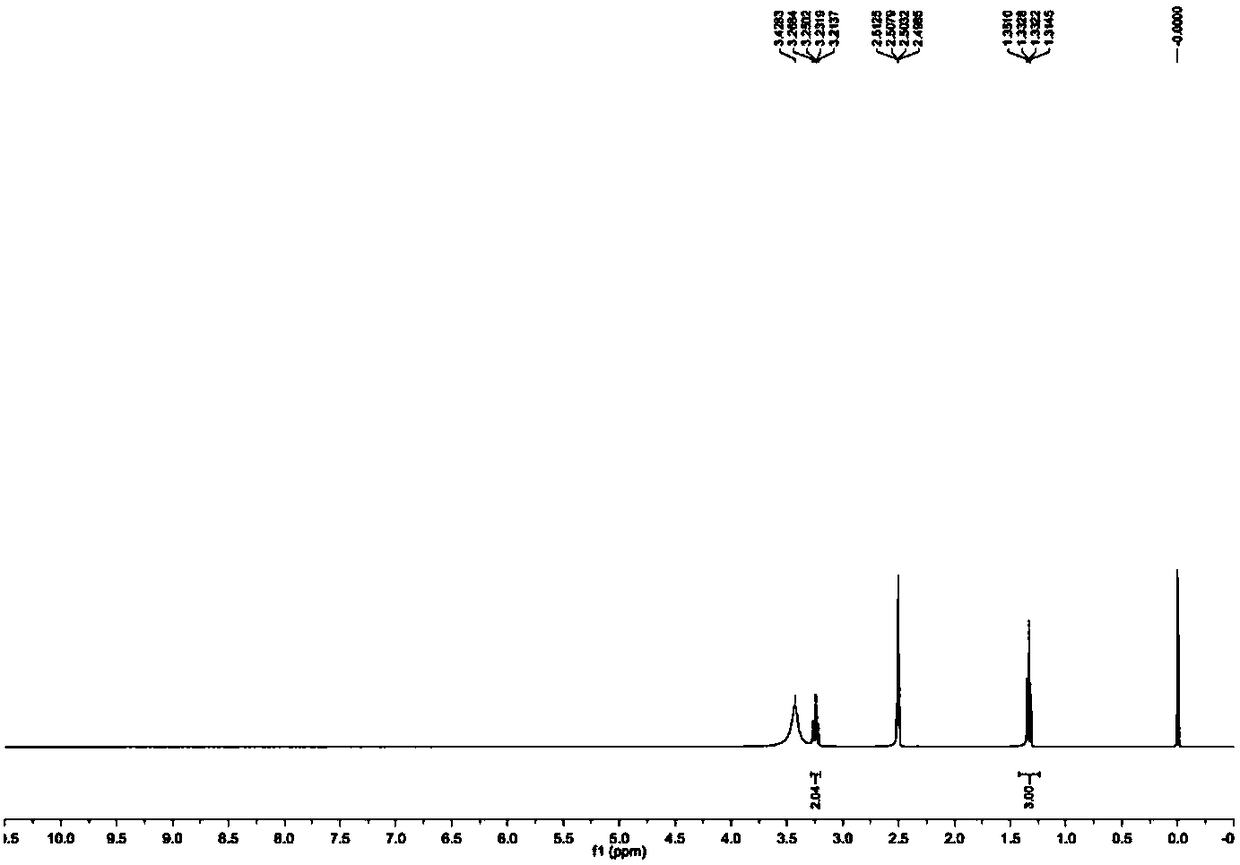
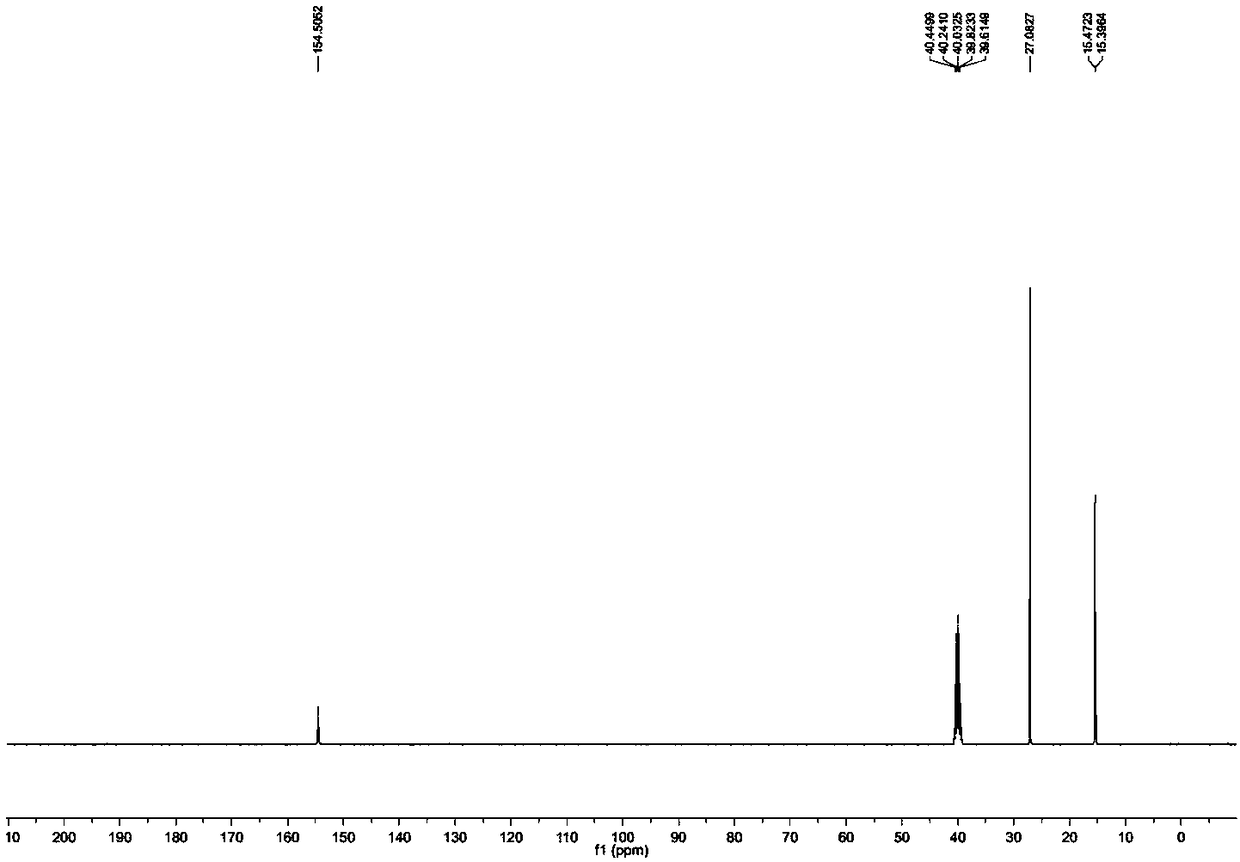
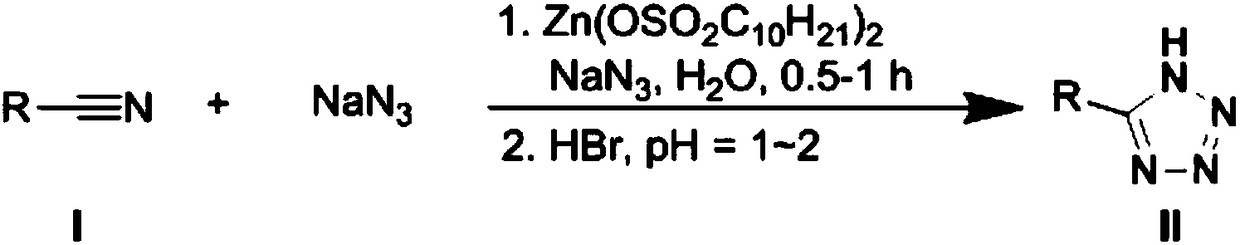Method for catalytically preparing 5'-substituted tetrazole compound by zinc Lewis acid surfactant
A technique for the preparation of surfactants and catalysis, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc. Low activity and other problems, achieve the effect of reducing catalyst dosage and reaction time, increasing reaction rate and conversion rate, improving solubility and dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] A kind of preparation method of 5'-substituted tetrazole compound II-1, its steps are:
[0055] A. Add ethyl thiocyanate I-1 (0.1mol, 8.7g), sodium azide (0.12mol, 7.8g) and water (300ml) in the reaction vessel, fully stir at room temperature to obtain a mixed solution;
[0056] B. Add zinc Lewis acid surfactant Zn(OSO 2 C 10 h 21 ) 2 (0.02mol, 10.0g),;
[0057] C. stirring reaction at room temperature, the reaction time is 0.5h;
[0058] D. Add hydrobromic acid dropwise to the mixed solution obtained in step C, adjust the pH to 1-2, centrifuge at 1000-1600rpm, and separate the mixed solution into a clear solution on the upper floor and a white turbid solution on the lower floor;
[0059] E. Cool the clear solution (upper layer) separated in step D to below 5°C, and recrystallize to obtain 12.0 g of 5′-ethylthiotetrazolium compound II-1, with a yield of 92%;
[0060] F. Cool the white turbid liquid (lower layer) separated in step D to below 10°C, and r...
Embodiment 2
[0065]
[0066] A kind of preparation method of 5'-substituted tetrazole compound II-2, its steps are:
[0067] A. Add methyl thiocyanate I-2 (0.1mol, 7.3g), sodium azide (0.12mol, 7.8g) and water (300ml) in the reaction vessel, fully stir at room temperature to obtain a mixed solution;
[0068] B. Add zinc Lewis acid surfactant Zn(OSO 2 C 10 h 21 ) 2 (0.02mol, 10.0g),;
[0069] C. stirring reaction at room temperature, the reaction time is 0.5h;
[0070] D. Add hydrobromic acid dropwise to the mixed solution obtained in step C, adjust the pH to 1-2, centrifuge at 1000-1600rpm, and separate the mixed solution into a clear solution on the upper floor and a white turbid solution on the lower floor;
[0071] E. Cool the clear solution (upper layer) separated in step D to below 5°C, and recrystallize to obtain 10.2 g of 5'-methylthiotetrazolium compound II-2, with a yield of 88%;
[0072] F. Cool the white turbid liquid (lower layer) separated in step D to below 10°C, and...
Embodiment 3
[0077]
[0078] A kind of preparation method of 5'-substituted tetrazole compound II-3, its steps are:
[0079] A. Add phenyl thiocyanate I-3 (0.1mol, 13.5g), sodium azide (0.12mol, 7.8g) and water (300ml) in the reaction vessel, stir fully at room temperature to obtain a mixed solution;
[0080] B. Add zinc Lewis acid surfactant Zn(OSO 2 C 10 h 21 ) 2 (0.02mol, 10.0g),;
[0081] C. stirring reaction at room temperature, the reaction time is 0.5h;
[0082] D. Add hydrobromic acid dropwise to the mixed solution obtained in step C, adjust the pH to 1-2, centrifuge at 1000-1600rpm, and separate the mixed solution into a clear solution on the upper floor and a white turbid solution on the lower floor;
[0083] E. Cool the clear solution (upper layer) separated in step D to below 5°C, and recrystallize to obtain 16.9 g of 5′-phenylthiotetrazolium compound II-3, with a yield of 95%;
[0084] F. Cool the white turbid liquid (lower layer) separated in step D to below 10°C, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com