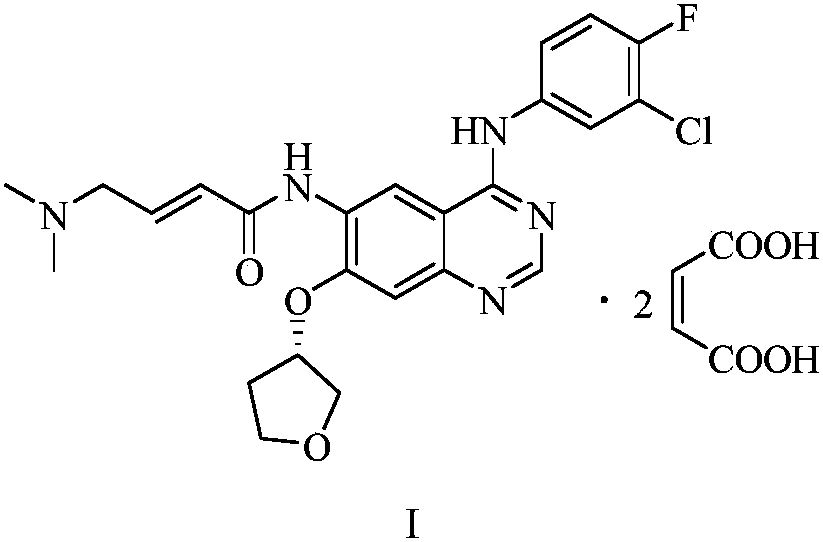
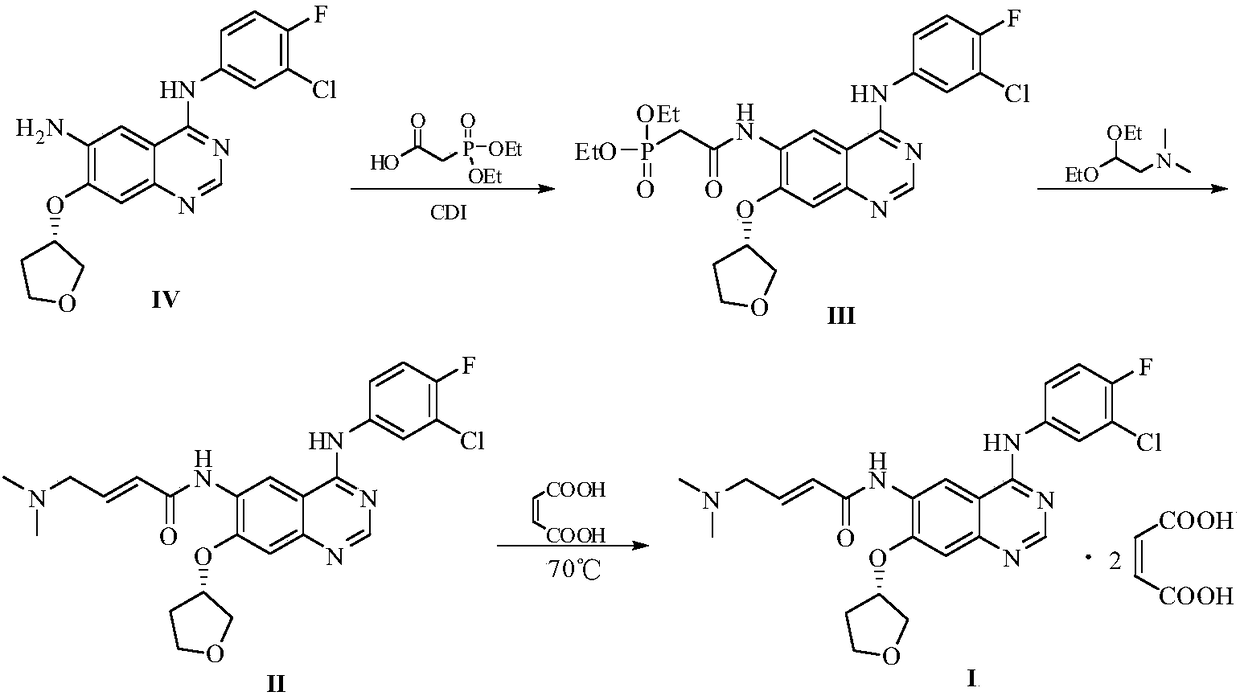
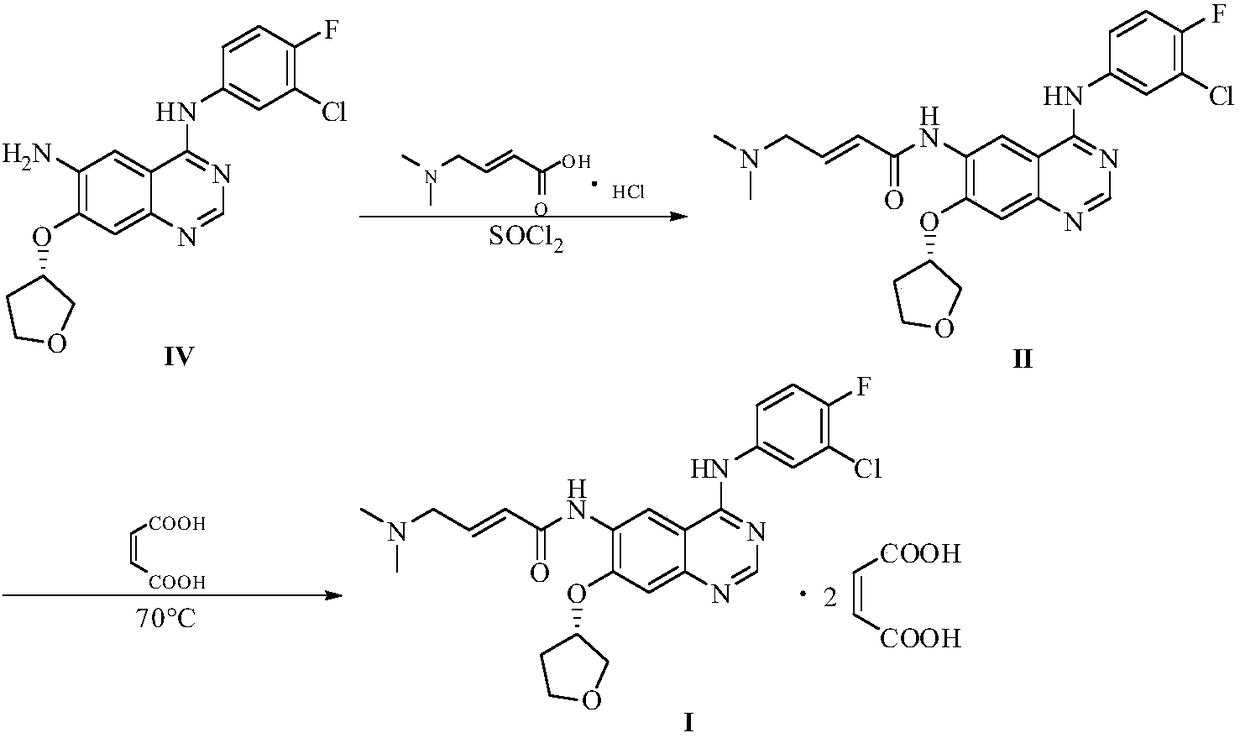
Preparation method of afatinib and maleate thereof
A technology of afatinib and afatinib maleate, which is applied in the field of drug synthesis, can solve problems such as hidden dangers of drug safety for patients, excessive content of impurity I, and impact on drug quality, and achieves simple operation, mild reaction conditions, and high purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of crude afatinib
[0021] Add 3.76kg of (E)-4-dimethylaminocrotonic acid hydrochloride and 36kg of N-methylpyrrolidone to a 100L reaction kettle, stir and cool to -5°C, and slowly add chlorine to the stirring solution. 2.56kg of sulfoxide to ensure that the temperature of the reaction solution is between 0 and -5°C, and stir for 20 minutes in the above temperature range after the dripping. Dissolve 4.85 kg of N-4-[(3-chloro-4-fluorophenyl)]-7-{[(3S)-tetrahydrofuran-3-yl]oxy}-4,6-quinazoline diamine in 24kg of N-methylpyrrolidone is used as a dropping liquid. Add this solution slowly to the above stirring liquid and ensure that the temperature is between 0~-5℃. After the dropping, stir for 15 minutes in the above temperature range to obtain the acylation reaction liquid. . Then add 10 kg of water dropwise, control the temperature not to exceed 15°C, transfer the above reaction liquid to a 500L reactor, slowly add 29 kg of water to the reaction solu...
Embodiment 2
[0022] Example 2: Preparation of crude afatinib
[0023] Add 19.3 g of (E)-4-dimethylamino crotonic acid hydrochloride and 185 g of N-methylpyrrolidone into a 1000 ml four-necked flask, stir and cool to -5°C, and slowly add dropwise to the stirring liquid 13.2g of thionyl chloride to ensure that the temperature of the reaction solution is between 0 and -5°C, and stir for 20 minutes in the above temperature range after the dripping is completed. Dissolve 24.9 g of N-4-[(3-chloro-4-fluorophenyl)]-7-{[(3S)-tetrahydrofuran-3-yl]oxy}-4,6-quinazoline diamine in 123g of N-methylpyrrolidone was used as a dropping solution, slowly adding this solution to the above stirring solution and ensuring that the temperature was between 0 to -5°C, and stirring within the above temperature range for 15 minutes after dropping to obtain an acylation reaction solution. Then, 200 g of water was slowly added dropwise to the above reaction solution and the temperature was controlled to not exceed 15°C, a...
Embodiment 3
[0026] Example 3: Preparation of crude afatinib
[0027] Add 9.65 g of (E)-4-dimethylamino crotonic acid hydrochloride and 93 g of N-methylpyrrolidone in a 500ml four-necked flask, stir and cool to -5°C, slowly add dropwise to the stirring solution 6.6 g of thionyl chloride to ensure that the temperature of the reaction solution is between 0 and -5°C, and stir for 20 minutes in the above temperature range after the dropping. Dissolve 12.45 g of N-4-[(3-chloro-4-fluorophenyl)]-7-{[(3S)-tetrahydrofuran-3-yl]oxy}-4,6-quinazoline diamine in 62g of N-methylpyrrolidone was used as a dropwise addition solution. The solution was slowly added dropwise to the above-mentioned stirring solution and the temperature was ensured to be between 0 and -5°C. After the dropping, the solution was stirred in the above-mentioned temperature range for 15 minutes to obtain an acylation reaction solution. Then, 100 g of water was slowly added dropwise to the above reaction solution and the temperature wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com