Fluorescent viologen derivative, application and preparation method thereof
A derivative and fluorescence technology, used in chemical instruments and methods, fluorescence/phosphorescence, color-changing fluorescent materials, etc., can solve the problems of poor stability of viologen cation free radicals, low fluorescence quantum yield, limited application and development, etc. Achieve the effect of good pH sensing, excellent fluorescence performance, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] In this example, see figure 1 and figure 2 , a preparation method of fluorescent viologen derivatives, comprising the steps of:
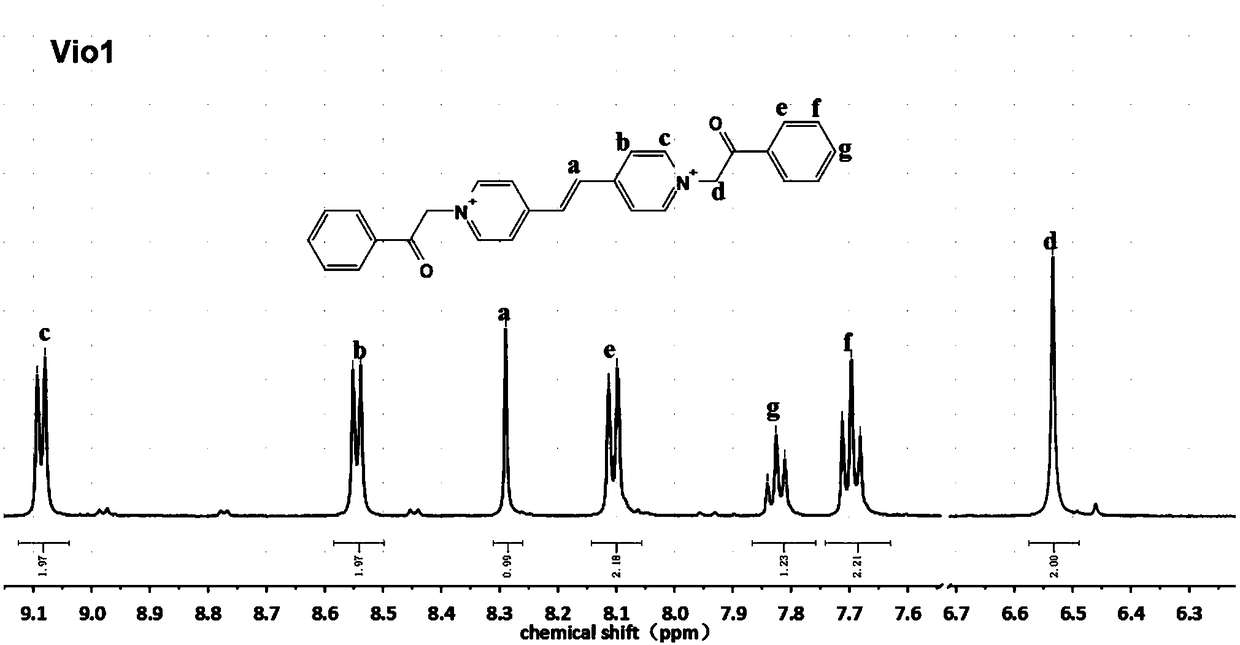
[0076] Weigh 2mmol (0.364g) of 1,2-bis(4-pyridine)ethylene (bpe) and 5mmol (0.773g) of 2-chloroacetophenone in a single-necked flask, add 6ml of anhydrous DMF to fully dissolve, and then The above mixture solution was refluxed at 120° C. for 12 h. During the reaction process, a light yellow precipitate was formed. After the reaction was completed, it was cooled to room temperature, and the mixture was centrifuged. The precipitate was washed with anhydrous DMF and acetone for 3 to 5 times, and the supernatant after centrifugation changed from brown to colorless. Vacuum After drying at 80° C. for 8 hours, 0.689 g of light yellow powder was collected to obtain vinyl viologen. The yield of fluorescent viologen derivative Vio1 calculated based on 1,2-bis(4-pyridyl)ethylene was 70.2%.
[0077] Experimental test analysis:
[0078] The fluoresce...
Embodiment 2
[0086] This embodiment is basically the same as Embodiment 1, especially in that:
[0087] In this example, see figure 1 and figure 2 , a preparation method of fluorescent viologen derivatives, comprising the steps of:
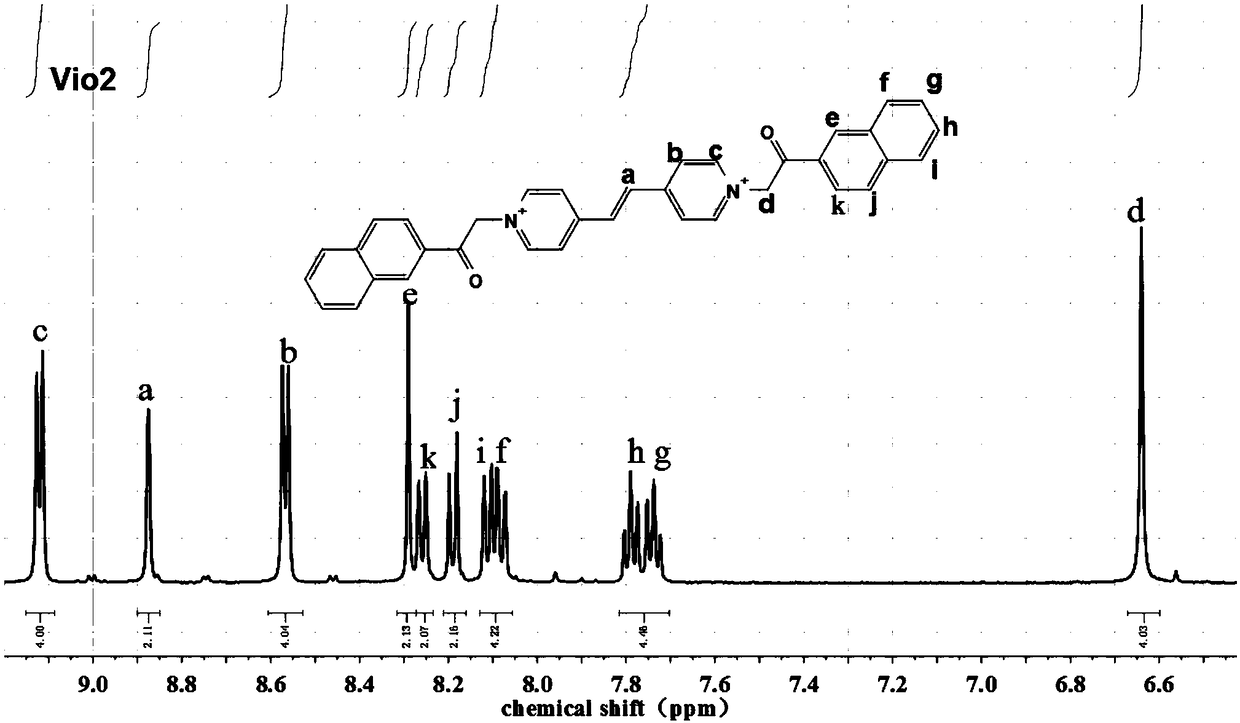
[0088] Weigh 2mmol (0.364g) of 1,2-bis(4-pyridine)ethylene (bpe) and 5mmol (0.773g) of 2-chloroacetophenone in a single-necked flask, add 6ml of anhydrous DMF to fully dissolve, and then The above mixture solution was refluxed at 120° C. for 12 h. During the reaction process, a light yellow precipitate was formed. After the reaction was completed, it was cooled to room temperature, and the mixture was centrifuged. The precipitate was washed with anhydrous DMF and acetone for 3 to 5 times, and the supernatant after centrifugation changed from brown to colorless. Vacuum After drying at 80° C. for 8 hours, 0.689 g of light yellow powder was collected to obtain vinyl viologen. The yield of fluorescent viologen derivative Vio2 calculated based on 1,2-bis(4-pyri...
Embodiment 3
[0098] This embodiment is basically the same as the previous embodiment, and the special features are:
[0099] In this example, see figure 1 and figure 2 , a preparation method of fluorescent viologen derivatives, comprising the steps of:
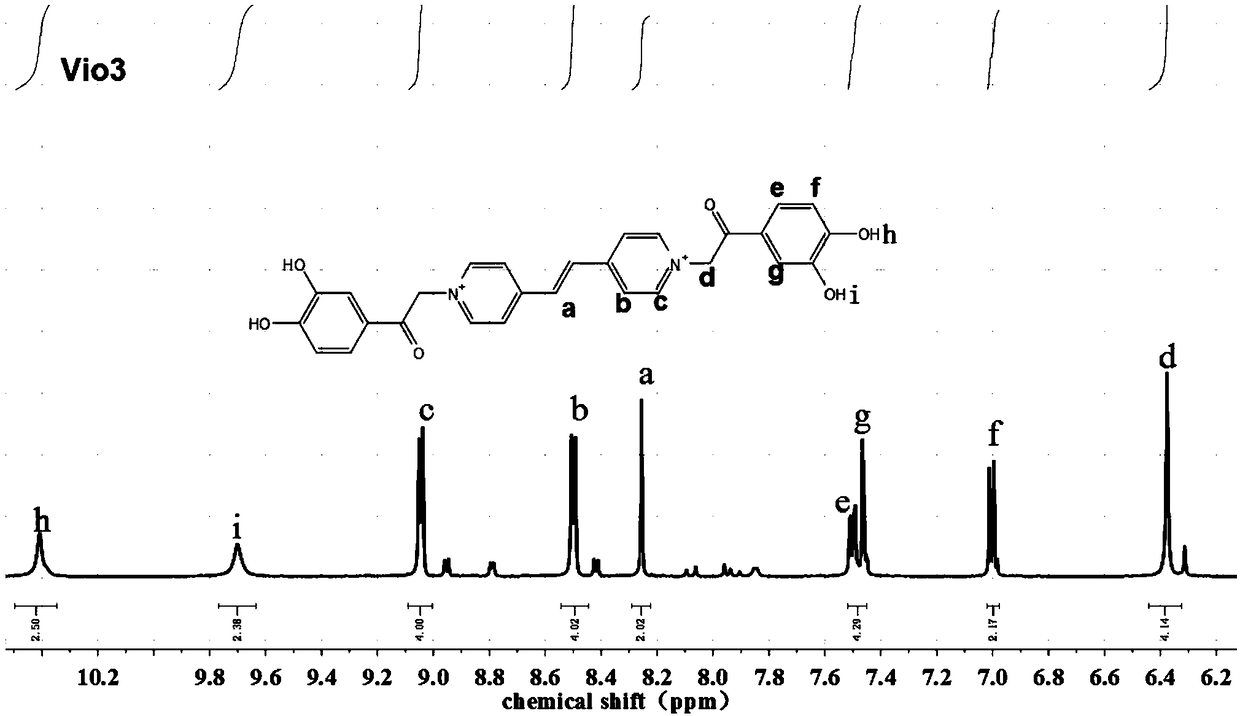
[0100] Weigh 2mmol / L (0.364g) of 1,2-bis(4-pyridine)ethylene (bpe) and 5mmol / L (0.933g) of 3,4-dihydroxy-2'-chloroacetophenone in a single-necked flask 10ml of anhydrous DMF was added to fully dissolve, and then the above mixture solution was refluxed at 120°C for 24h. During the reaction process, a yellow precipitate was formed. After the reaction was completed, cool to room temperature, centrifuge the mixture, and wash the precipitate with anhydrous DMF and acetone for 3 to 5 times, until the supernatant after centrifugation changed from brown to colorless, vacuum 80 After drying at °C for 8 hours, 0.759 g of reddish-brown powder was collected to obtain vinyl viologen. The yield of fluorescent viologen derivative Vio3 calculated base...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com