Co-production method of trans-1-chlorine-3,3,3-trifluoropropene and 2,3,3,3-tetrafluoropropene
A technology of trifluoropropene and tetrafluoropropene, which is applied in the field of preparation of fluorine-containing olefins and fluorine-containing chloroolefins, can solve the problems of excessive three wastes, bulging, and high synthesis cost, and achieves simple processing technology, high operating flexibility, and low loss live effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
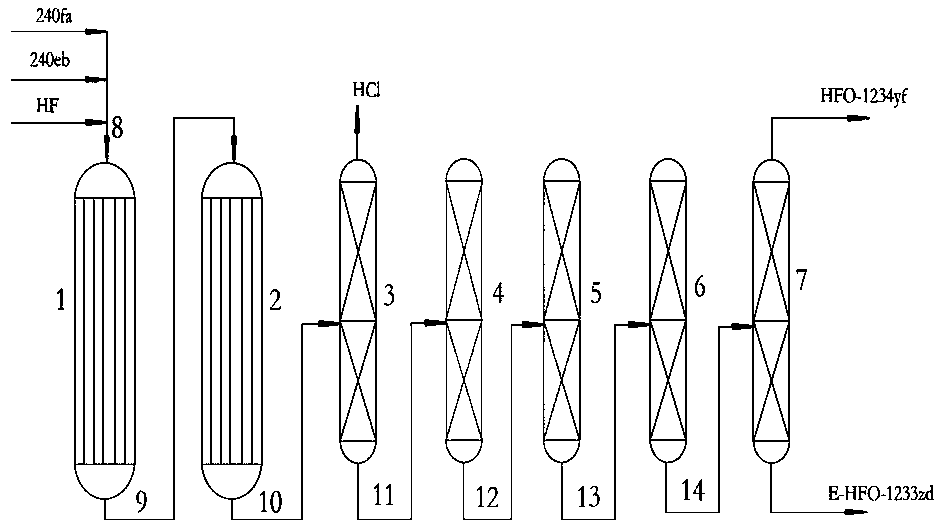
Image
Examples
Embodiment 1
[0033] 10ml of chromium oxide + alumina, loaded with cobalt and nickel, the loading of cobalt is 2wt%, and the loading of nickel is 10wt%. The catalyst is loaded into the first reactor, and 10ml of chromium oxide is loaded with gallium, and the loading of gallium is 5wt%. Load the catalyst into the second reactor, raise the temperature to a bed temperature of 350°C, pass HF into it for activation, the flow rate of HF: 10g / h, the hot spot temperature <400°C, and fluoride for 40 hours.
[0034] The first reactor is fed with HF, 1,1,1,3,3-pentachloropropane and 1,1,1,2,3-pentachloropropane, the relevant reaction conditions and the composition of the second reactor outlet, the results are shown in the table 1.
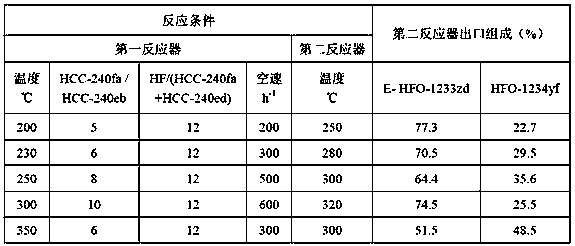
[0035] Table 1: Example 1 reaction conditions and the composition of the second reactor outlet
[0036]
Embodiment 2
[0038] 10ml of chromium oxide + alumina, loaded with cobalt and nickel, the loading of cobalt is 5wt%, and the loading of nickel is 12wt%. The catalyst is loaded into the first reactor, and 10ml of chromium oxide is loaded with gallium, and the loading of gallium is 10wt%. Load the catalyst into the second reactor, raise the temperature to a bed temperature of 350°C, pass HF into it for activation, the flow rate of HF: 10g / h, the hot spot temperature <400°C, and fluoride for 40 hours.
[0039] The first reactor is fed with HF, 1,1,1,3,3-pentachloropropane and 1,1,1,2,3-pentachloropropane, the relevant reaction conditions and the composition of the second reactor outlet, the results are shown in the table 2.
[0040] Table 2: Embodiment 2 reaction conditions and the composition of the second reactor outlet
[0041]
Embodiment 3
[0043] 10ml of chromium oxide + alumina, loaded with cobalt and nickel, the loading of cobalt is 3wt%, and the loading of nickel is 15wt%. The catalyst is loaded into the first reactor, and 10ml of chromium oxide is loaded with gallium, and the loading of gallium is 8wt%. Load the catalyst into the second reactor, raise the temperature to a bed temperature of 350°C, pass HF into it for activation, the flow rate of HF: 10g / h, the hot spot temperature <400°C, and fluoride for 40 hours.
[0044] The first reactor is fed with HF, 1,1,1,3,3-pentachloropropane and 1,1,1,2,3-pentachloropropane, the relevant reaction conditions and the composition of the second reactor outlet, the results are shown in the table 3.
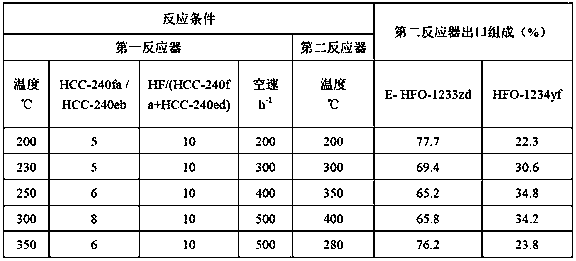
[0045] Table 3: Embodiment 3 reaction conditions and the composition of the second reactor outlet
[0046]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com