A kind of method utilizing synthetic triacetone amine process by-product to prepare acetone
A technology of triacetoneamine and by-products, applied in the field of preparation of acetone, can solve problems such as yield affecting reaction conversion, environmental pollution, and synthesis process conditions cannot be satisfied at the same time, and achieve the effects of improving yield and reducing comprehensive production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
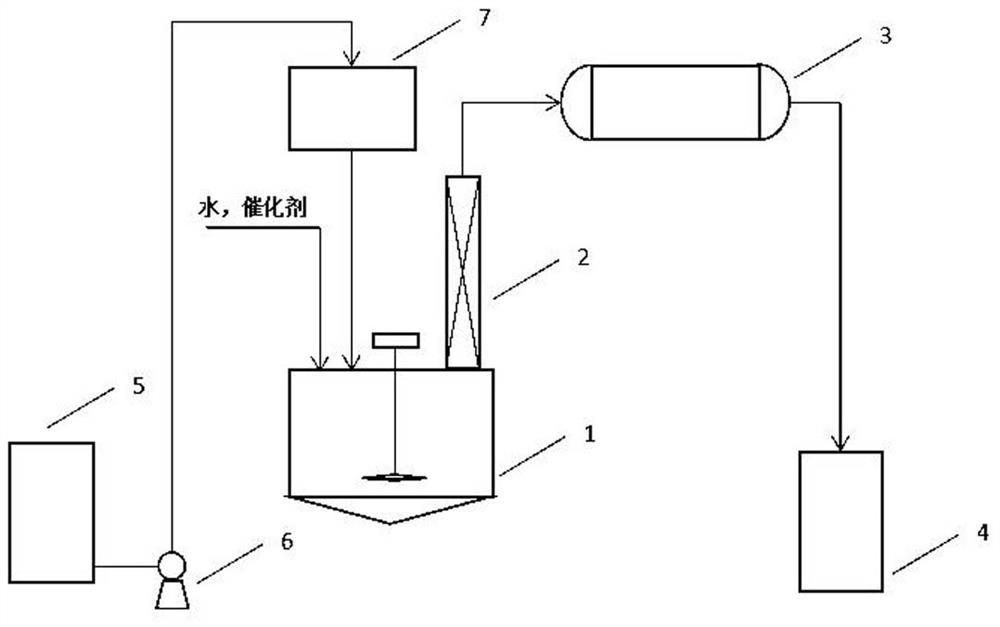
[0026] In the first step, 3000kg of by-products are poured into the high-temperature tank, and the by-products are analyzed by gas chromatography and composed of: 3.57% acetone, 42.03% mesityl oxide, 1.68% diacetone alcohol, 0.19% diacetone amine, DHP (2, 2,4,6-tetramethyl-2,3-dihydropyridine) 17.6%, acetonin 31.4%, phorone 0.17%, triacetonamine 1.24%, other components 2.12%;
[0027] Second step, add decyl diamine 300kg, water 3000kg in reactor;
[0028] The third step is to start stirring and heat the kettle to 85°C;
[0029] The fourth step is to drop by-products into the kettle and keep the temperature of the kettle at 85°C;
[0030] The 5th step, after by-product dropwise addition, obtain regenerated acetone 2196kg, analyze acetone 97.30% through gas chromatography, analyze water content 1.84% through Karl Fischer method, still liquid is formed through gas chromatography analysis: acetone 5.71%, isopropyl 0.34% diacetone, 85.26% DHP, 1.06% acetonin, 1.69% triacetonamine...
Embodiment 2
[0032] In the first step, 3000kg of by-products are poured into the high-temperature tank, and the by-products are analyzed by gas chromatography and composed of: 3.57% acetone, 42.03% mesityl oxide, 1.68% diacetone alcohol, 0.19% diacetone amine, DHP (2, 2,4,6-tetramethyl-2,3-dihydropyridine) 17.6%, acetonin 31.4%, phorone 0.17%, triacetonamine 1.24%, other components 2.12%;
[0033] In the second step, add 600kg of secondary amino-based cation exchange resins, 3000kg of water in the reactor;
[0034] The third step is to start stirring and heat the kettle to 90°C;
[0035] The fourth step is to drop by-products into the kettle and keep the temperature of the kettle at 90°C;
[0036] The 5th step, after by-product dropwise addition, obtain regenerated acetone 2015kg, analyze acetone 97.13% through gas chromatography, analyze water content 1.79% through Karl Fischer method, still liquid is formed through gas chromatography analysis: acetone 4.27%, isopropyl 7.14% triacetone,...
Embodiment 3
[0038] In the first step, 3000kg of by-products are poured into the high-temperature tank, and the by-products are analyzed by gas chromatography and composed of: 3.57% acetone, 42.03% mesityl oxide, 1.68% diacetone alcohol, 0.19% diacetone amine, DHP (2, 2,4,6-tetramethyl-2,3-dihydropyridine) 17.6%, acetonin 31.4%, phorone 0.17%, triacetonamine 1.24%, other components 2.12%;
[0039] In the second step, add 900kg of porous alumina loaded with potassium carbonate in the reactor, and 3000kg of water;
[0040] The third step is to start stirring and heat the kettle to 95°C;
[0041] The fourth step is to drop by-products into the kettle and keep the temperature of the kettle at 95°C;
[0042] The 5th step, after by-product dropwise addition, obtain regenerated acetone 1857kg, analyze acetone 97.13% through gas chromatography, analyze water content 2.03% through Karl Fischer method, still liquid is formed through gas chromatography analysis: acetone 5.34%, isopropyl 8.59% triac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com