Monosubstituted cobaltocene cationic derivative and high-efficiency preparation method
A cobaltocene and single-substitution technology, which is applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems of few reports on cobaltocene derivatives, achieve simple and fast post-treatment, high reaction efficiency, Simple and easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
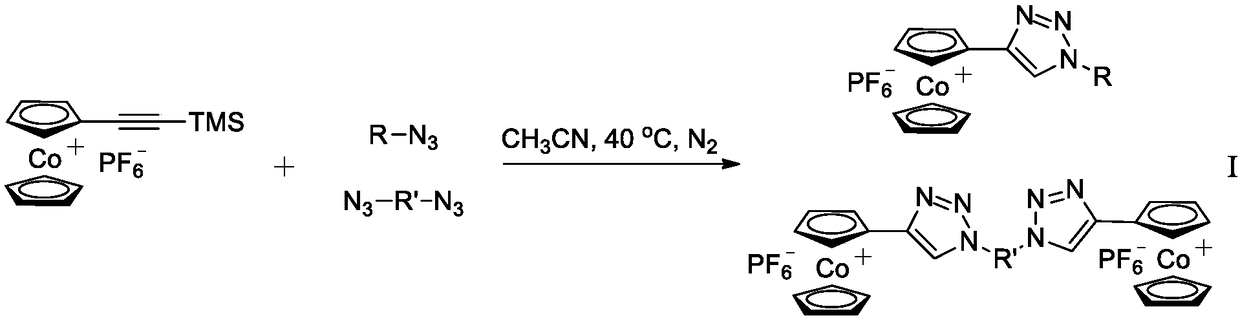
[0038] The preparation method of two kinds of compounds represented by general formula I, according to figure 1 The compound reacts according to the specified route to obtain one of the following general formulas:
[0039]
[0040] In the reaction, R=-C 12 h 25 ,-C 14 h 29 ,-C 16 h 33 ,-(CH 2 ) 11 OOC (C 5 h 5 ) 2 Fe,-(CH 2 ) 2 OH; R'=-CH 2 C(CH 2 Oh) 2 CH 2 -, -C 12 h 24 -, TMS means trimethylsilyl.
Embodiment 1
[0041] Embodiment 1: when R=-C 12 h 25 preparation of
[0042] Dissolve 2.32 mmol of trimethylsilyl-protected alkynyl cobaltocene cobalt hexafluorophosphate and 1.06 mmol of bis-azido-neopentyl glycol in 20 mL of anhydrous acetonitrile, and deoxygenate with nitrogen for 30 min; at the same time, in another round-bottomed flask Add 0.2mmol cuprous iodide (CuI) to the solution, pass nitrogen gas to remove oxygen for 30min; then transfer the acetonitrile solution to the flask of CuI under the protection of nitrogen, add 10.6mmol finely ground anhydrous potassium carbonate after 5min, and protect it under nitrogen at 40°C Under reaction 24h. The reacted suspension was filtered, rinsed with acetonitrile, and then concentrated by rotary evaporation. The concentrated solution was added dropwise to absolute ethanol, and the precipitate was collected and dried in a vacuum oven for 24 hours to obtain 81% of compound I-1.
[0043] 1 H NMR (d 6 -DMSO, δ / ppm) 3.28 (m, 4H), 4.54 (s, 4H...
Embodiment 2
[0049] Embodiment 2, the R=-C 14 h 29 When the preparation
[0050] Dissolve 3.262 mmol of trimethylsilyl-protected alkynyl cobaltocene cobalt hexafluorophosphate and 2.965 mmol of 1-azidodecane in 20 mL of anhydrous acetonitrile, and deoxygenate with nitrogen for 30 min; at the same time, in another round-bottomed flask Add 0.6mmol cuprous iodide (CuI), pass nitrogen gas to remove oxygen for 30min; then transfer the acetonitrile solution to the flask of CuI under nitrogen protection, add 29.6mmol finely ground anhydrous potassium carbonate after 5min, at 40°C under nitrogen protection Reaction 24h. After the reaction, the suspension was filtered, rinsed with acetonitrile, and then concentrated by rotary evaporation. The concentrated solution was added dropwise to anhydrous ether, and the precipitate was collected and dried in a vacuum oven for 24 hours to obtain 80% of compound I-2.
[0051] 1 H NMR (d 6 -DMSO,δ / ppm)0.86(t,3H),1.22(m,18),1.86(t,2H),4.42(t,2H),5.67(s,5H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com