Method of tin-graphene airflow-coated high-nickel ternary lithium battery electrode material
A ternary lithium battery and electrode material technology, applied in battery electrodes, electrical components, secondary batteries, etc., can solve the problems of limited performance improvement and lower specific capacity, and achieve high production capacity, high activity, and continuous production process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
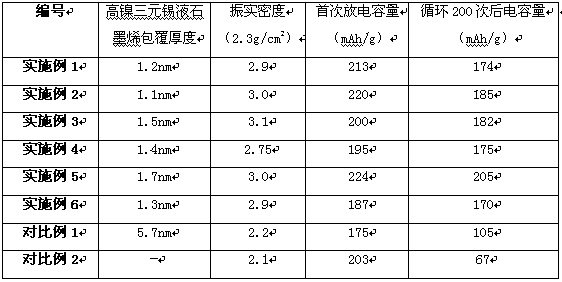
Embodiment 1
[0038] (1). Prepare 10L nickel nitrate containing 1.4molNi(NO 3 ) 2 , 0.2mol cobalt nitrate Co(NO 3 ) 2 , 0.3mol manganese nitrate Mn (NO 3 ) 2 , 2mol lithium nitrate LiNO 3 The mixed solution of nitrates was sprayed and dried to obtain the premix.
[0039] (2). Calcinate the premixed material obtained in step (1) at 750°C for 0.9h, and perform jet milling on a fluidized bed to obtain nanoparticles A. The gas used in the jet mill is compressed air;
[0040] (3). Mix 0.02kg metal tin and 1.5Kg graphene under the protection of nitrogen, heat the metal tin and graphene to 235°C for 5 minutes under nitrogen atmosphere, and obtain tin liquid B;
[0041] (4). Put 1Kg of nanoparticles A in step (2) and 0.04Kg of tin liquid B in step (3) into two storage tanks opposite to the high-speed airflow collider, and use nitrogen with a pressure of 0.3Mpa as the delivery gas. The nanoparticles A and tin liquid B are collided in the airflow collider in the form of gas fluid. The flow vel...
Embodiment 2
[0043] (1). Prepare 10L nickel nitrate containing 1.6molNi(NO 3 ) 2 , 0.2mol cobalt nitrate Co(NO 3 ) 2 , 0.3mol manganese nitrate Mn (NO 3 ) 2 , 2.2mol lithium nitrate LiNO 3 The mixed solution of nitrates was sprayed and dried to obtain the premix.
[0044] (2). Calcinate the premix obtained in step (1) at 760° C. for 0.9 h, and perform jet milling on a fluidized bed to obtain nanoparticles A. The gas used in the jet mill is compressed air;
[0045] (3). Mix 0.03kg metal tin and 1.5Kg graphene under the protection of nitrogen, heat the metal tin and graphene to 240°C for 6 minutes under nitrogen atmosphere, and obtain tin liquid B;
[0046] (4). Put 1Kg of nanoparticles A in step (2) and 0.04Kg of tin liquid B in step (3) into two storage tanks opposite to the high-speed airflow collider, and use nitrogen with a pressure of 0.4Mpa as the delivery gas. The nanoparticles A and tin liquid B are collided in the airflow collider in the form of gas fluid. The flow velocity ...
Embodiment 3
[0048] (1). Prepare 10L nickel nitrate containing 1.7molNi(NO 3 ) 2 , 0.2mol cobalt nitrate Co(NO 3 ) 2 , 0.4mol manganese nitrate Mn (NO 3 ) 2 , 2.4mol lithium nitrate LiNO 3 The mixed solution of nitrates was sprayed and dried to obtain the premix.
[0049] (2). The premixed material obtained in step (1) was roasted at 770°C for 0.9h, and jet milled in a fluidized bed to obtain nanoparticles A. The gas used in the jet mill was compressed air;
[0050] (3). Mix 0.02kg metal tin and 1.7Kg graphene under the protection of nitrogen, heat the metal tin and graphene to 300°C for 5 minutes under nitrogen atmosphere, and obtain tin liquid B;
[0051] (4). Put 1.5Kg of nanoparticles A in step (2) and 0.03Kg of tin liquid B in step (3) into two storage tanks opposite to the high-speed airflow collider, and use nitrogen with a pressure of 0.4Mpa as the delivery gas , the nanoparticle A and tin liquid B are collided in the airflow collider in the form of gas fluid. The flow veloc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com