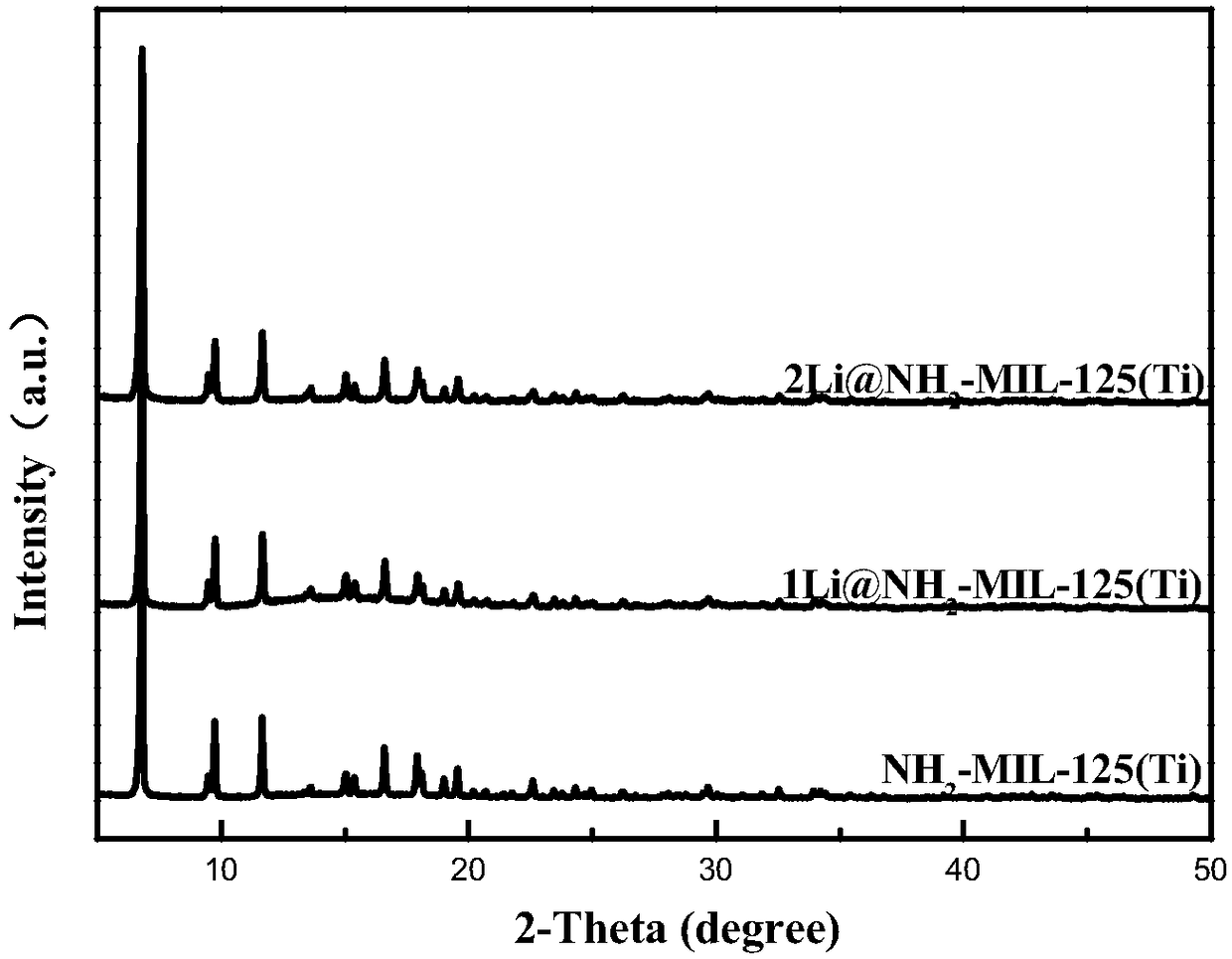
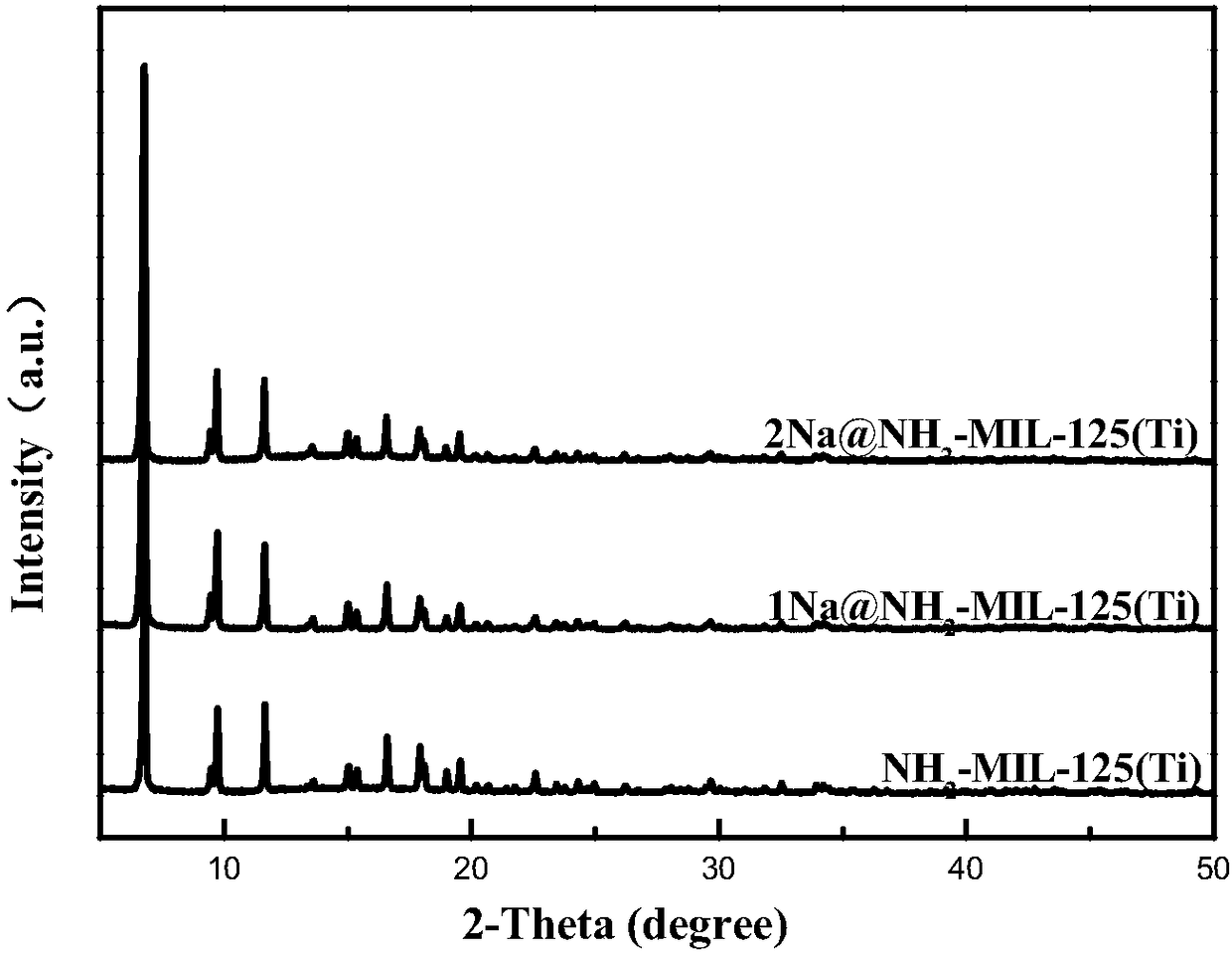
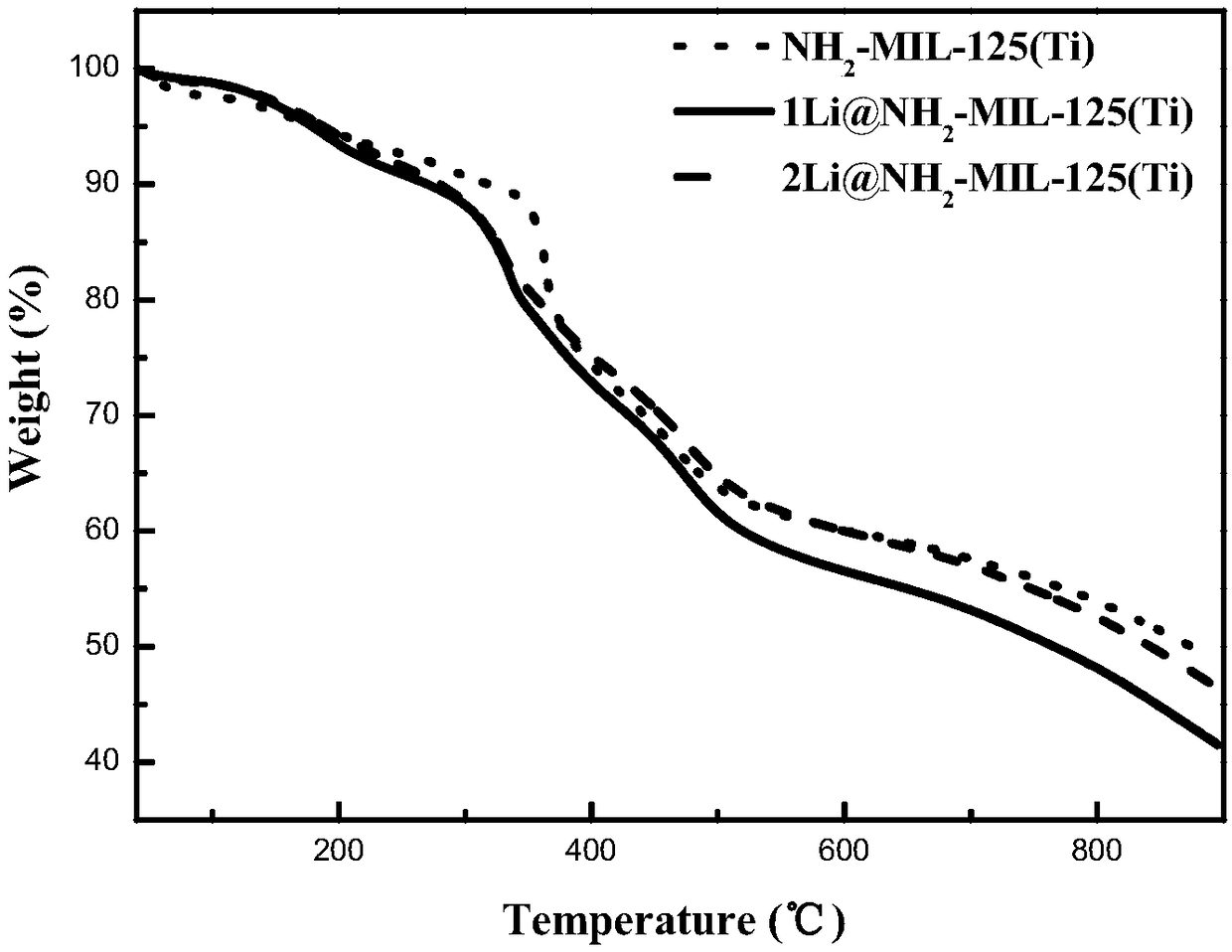
Alkali metal cation doping-based NH1-MIL-125 (Ti) material and preparation method thereof
A technology of NH2-MIL-125 and alkali metal cations, applied in the direction of alkali metal compounds, alkali metal oxides/hydroxides, chemical instruments and methods, etc., can solve the problem of unstable impregnation environment, large influence on samples, and preparation cycle Long and other problems, to achieve the effect of shortening the synthesis time, increasing the reaction temperature, and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step 1) The solute ratio in the solvent is 8g / mL, and the volume ratio of 45% N,N-dimethylformamide and 55% methanol is stirred at room temperature for 10 minutes to obtain a mixed organic solvent;
[0043] Step 2) Weighing 2-aminoterephthalic acid with a mass fraction of 56 wt%, slowly adding it to the prepared organic solvent, and stirring for 20 minutes to obtain a mixed solution of nitrogen-containing ligands;
[0044] Step 3) adding the dry chlorine salt containing alkali metal cations, lithium chloride with a mass fraction of 1 wt% to the solvent prepared in step 2, and stirring at room temperature for 20 min to obtain a mixed solution containing alkali metal cations and nitrogen ligands ;
[0045] Step 4) Slowly add 43wt% titanium isopropoxide into the mixed solution under the condition of ice-water bath, and the stirring time is 30 minutes to obtain a uniformly mixed reddish-brown liquid;
[0046] Step 5) Transfer the uniformly stirred mixture into a 40mL polyt...
Embodiment 2
[0048] Step 1) The solute ratio in the solvent is 8g / mL, and the volume ratio of 45% N,N-dimethylformamide and 55% methanol is stirred at room temperature for 10 minutes to obtain a mixed organic solvent;
[0049] Step 2) Weighing 2-aminoterephthalic acid with a mass fraction of 56 wt%, slowly adding it to the prepared organic solvent, and stirring for 20 minutes to obtain a mixed solution of nitrogen-containing ligands;
[0050] Step 3) adding the dry chloride salt containing alkali metal cations, sodium chloride with a mass fraction of 1 wt% to the solvent prepared in step 2, and stirring at room temperature for 20 min to obtain a mixed solution containing alkali metal cations and nitrogen ligands ;
[0051] Step 4) Slowly add 43wt% titanium isopropoxide into the mixed solution under the condition of ice-water bath, and the stirring time is 30 minutes to obtain a uniformly mixed reddish-brown liquid;
[0052] Step 5) Transfer the uniformly stirred mixture into a 40mL polyte...
Embodiment 3
[0054] Step 1) The solute ratio in the solvent is 8g / mL, and the volume ratio of 45% N,N-dimethylformamide and 55% methanol is stirred at room temperature for 10 minutes to obtain a mixed organic solvent;
[0055] Step 2) Weighing 2-aminoterephthalic acid with a mass fraction of 55wt%, slowly adding it into the prepared organic solvent, and stirring for 20 minutes to obtain a mixed solution of nitrogen-containing ligands;
[0056] Step 3) adding the dry chlorine salt containing alkali metal cations, lithium chloride with a mass fraction of 2wt% to the solvent prepared in step 2, and stirring at room temperature for 20 min to obtain a mixed solution containing alkali metal cations and nitrogen ligands ;
[0057] Step 4) Slowly add 43wt% titanium isopropoxide into the mixed solution under the condition of ice-water bath, and the stirring time is 30 minutes to obtain a uniformly mixed reddish-brown liquid;
[0058] Step 5) Transfer the uniformly stirred mixture into a 40mL polyt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com