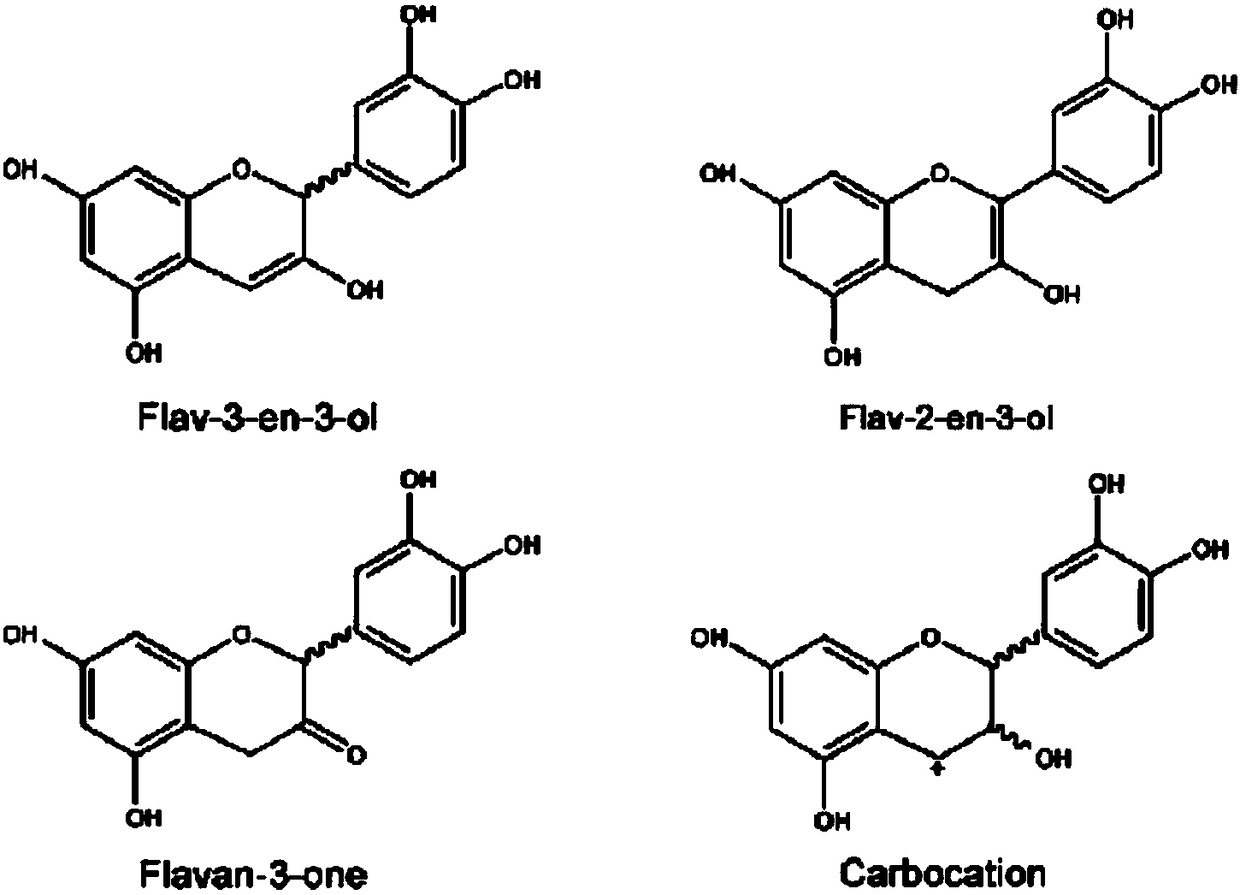
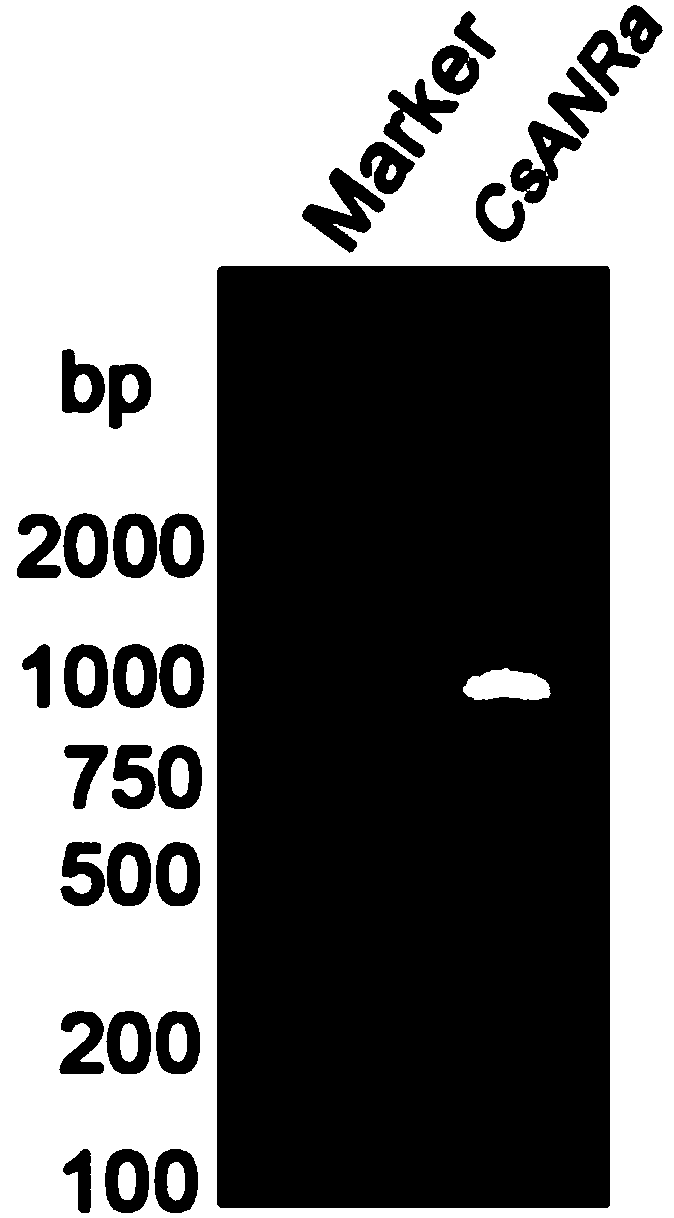
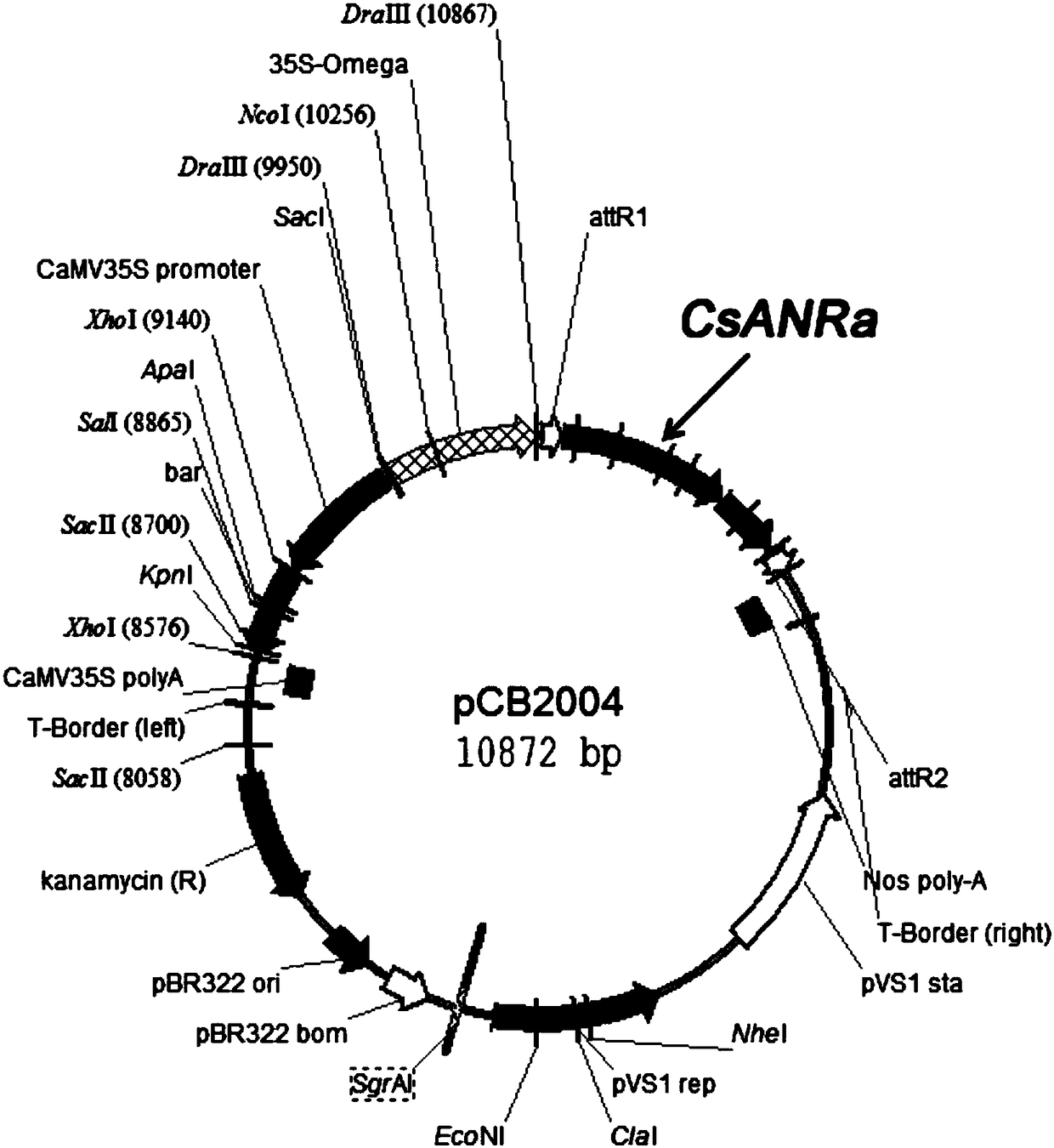
Methods for biologically synthesizing and detecting catechin intermediates
A technology for biosynthesis and intermediates, applied in the fields of biochemical equipment and methods, botanical equipment and methods, introduction of foreign genetic material using vectors, etc., can solve uncertain, no intermediate synthesis and detection, extraction and detection of intermediates The method is not perfect and other problems, to achieve the effect of simple operation, low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Materials
[0027] 1.1. Tea tree species: Camellia sinensis (L.) O.Kuntze.var.sinensiscultivar Nongkangzao), fresh tea leaves were collected, quickly frozen with liquid nitrogen, and stored in a -80°C refrigerator for later use;
[0028] 1.2. Escherichia coli competent Trans T1: purchased from Beijing Quanshijin Biotechnology Co., Ltd.;
[0029] 1.3. Agrobacterium Competent EHA105: gifted by University of Science and Technology of China, or purchased directly;
[0030] 1.4. High-fidelity enzyme Phusion High-Fidelity DNA Polymerase: purchased from Thermo Company; plasmid extraction kit and gel recovery extraction kit were purchased from Beijing Aidelai Company (Beijing, China).
[0031] 1.5. Medium
[0032] 1.5.1. LB medium: Weigh 10g of NaCl, 5g of yeast extract, and 10g of tryptone, add 950 mL of ultrapure water and stir to dissolve, adjust the pH to 7.0 with 1mol / L NaOH, add water to volume 1000mL, sterilized by high-pressure steam at 120°C for 21 minutes to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com