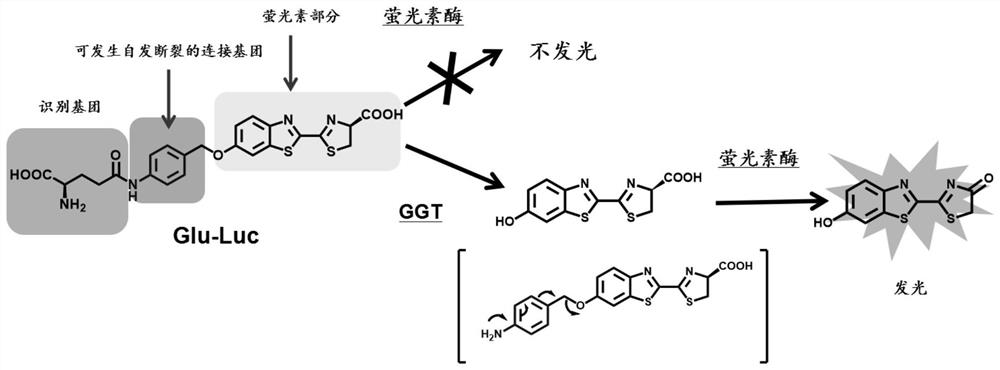
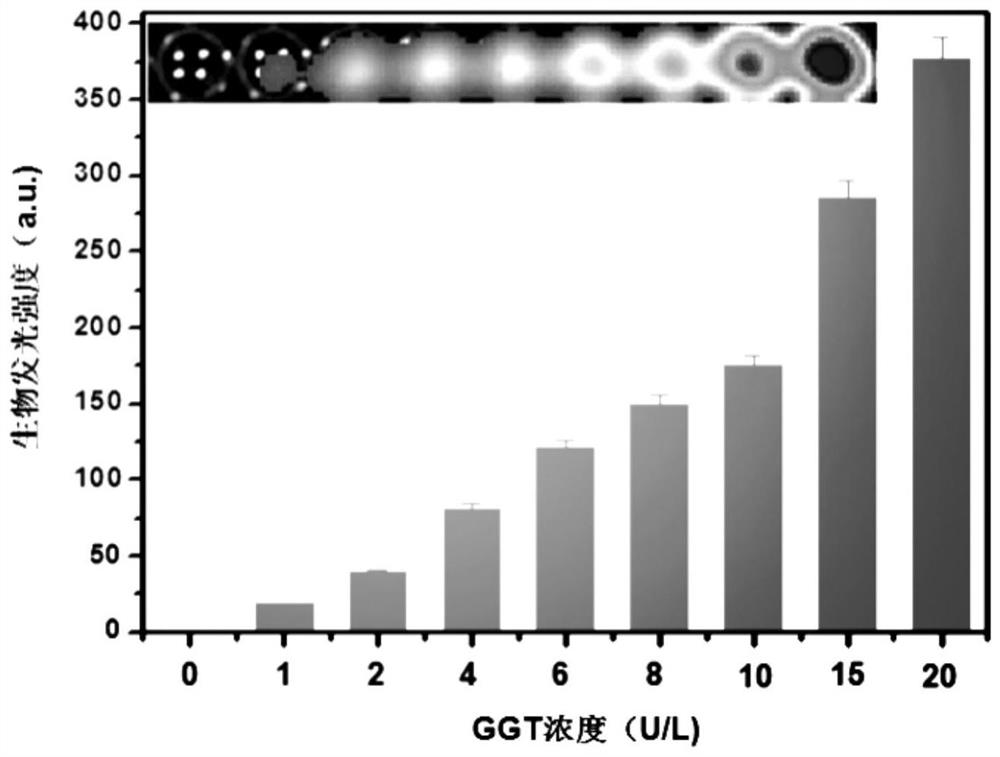
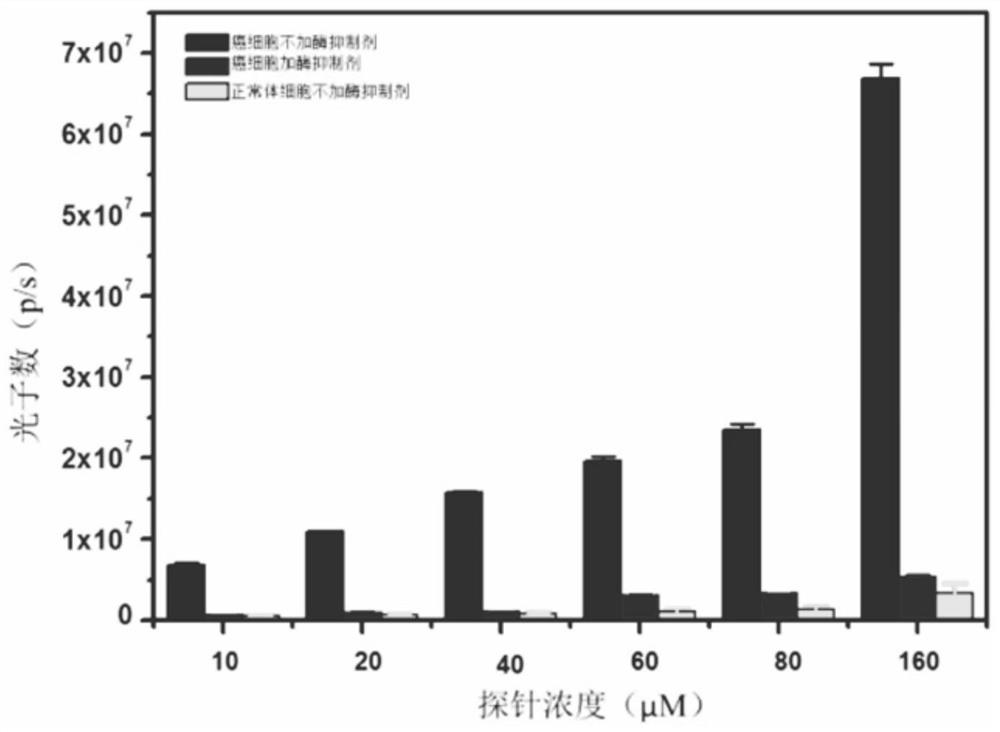
γ-glutamyl transpeptidase biological probe and its preparation method and application
A technology of reaction and application, applied in the field of γ-glutamyl transpeptidase biological probe and its preparation and application, can solve the problems of high detection cost, large consumption of organic solvent, large environmental pollution, etc., and achieves good selectivity and high efficiency. Sensitivity, fewer synthesis steps, and shorter reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of compound (1)
[0060]
[0061] Under argon protection, N-BOC-L-glutamic acid-1-tert-butyl ester (3.3mmol, 1.01g) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (3.3mmol, 632.6mg) was dissolved in 6ml of dichloromethane, and a solution of 4-aminobenzyl alcohol (3.0mmol, 369mg) dissolved in 4mL of dichloromethane was slowly added dropwise at 0°C, and stirred at room temperature for 1.5h . After the reaction was completed, the organic phase was spin-dried, and the crude product was passed through a silica gel column with a developing solvent of ethyl acetate / petroleum ether / methanol to obtain a white solid (945mg, 78%). ESI (C 21 h 32 N 2 o 6 ):[M+23] + = 431.4. 1 H-NMR (400MHz, CD 4 O), δ: 7.54(d, J=8.4, 2H), 7.31(d, J=8.4, 2H), 4.57(s, 2H), 4.05-4.01(m, 1H), 2.46(t, J=7.6 ,2H),2.23-2.15(m,1H),2.03-1.94(m,1H),1.49(s,9H),1.45(s,9H).
Embodiment 2
[0063] Preparation of compound (2)
[0064]
[0065] Under argon protection, compound 1 (1.16mmol, 500mg) and triethylamine (1.27mmol, 0.3ml) were dissolved in 10ml of dichloromethane, and p- Tosyl chloride (2.52mmol, 480mg), continued stirring for 2.5h. After the reaction was completed, the organic phase was spin-dried, and the crude product was passed through a silica gel column with the developer ethyl acetate / petroleum ether to obtain a white solid intermediate (690 mg, 46%). Under argon protection, the intermediate product (1.2mmol, 660mg), 2-cyano-6-hydroxybenzothiazole (1.4mmol, 246.7mg), potassium carbonate (7.1mmol, 981.3mg), sodium iodide (0.4 mmol, 59.9mg) was dissolved in 12mL of acetonitrile and reacted at 85°C for 5h. After the reaction was completed, the reaction solution was filtered, the organic phase was extracted with dichloromethane, and the organic phase was spin-dried, and the crude product was passed through a silica gel column with a developer ethy...
Embodiment 3
[0067] Preparation of compound (3)
[0068]
[0069] Under argon protection, compound 2 (0.42mmol, 240mg) was dissolved in a mixed solvent of 5ml methanol and dichloromethane (v / v=1:1), and added with a mixed solvent of 5ml methanol and deionized water (v / v=1:1) solution of dissolved D-cysteine hydrochloride (0.45 mmol, 55 mg) and potassium carbonate (0.45 mmol, 62 mg). React at room temperature for 20 minutes. After the reaction, the organic solvent was spin-dried, an equal volume of water was added, and the pH was adjusted to about 2-3 with 1M hydrochloric acid solution. The crude product obtained was a yellow solid. The crude product was recrystallized from methanol to give a slightly yellow product (100 mg, 53.9%). ESI (C 32 h 38 N 4 o 8 S 2 ):[M-1] — = 669.8. 1 H-NMR (300MHz, DMSO), δ: 9.96(s, 1H), 8.04(d, J=9.0Hz, 1H), 7.84(s, 1H), 7.60(d, J=8.3Hz, 2H), 7.40 (d, J=8.4Hz, 2H), 7.30–7.19(m, 1H), 7.13(d, J=7.4Hz, 1H), 5.39(t, J=9.0Hz, 1H), 5.12(s, 2H) ,4.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com