Epirubicin VES (vitamin E succinate) compound and preparation method and application thereof
A technology of epirubicin and compound, which is applied in the field of epirubicin VES compound, can solve the problems of drug efficacy, toxicity and side effects, and achieve the effect of high activity and reduced toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
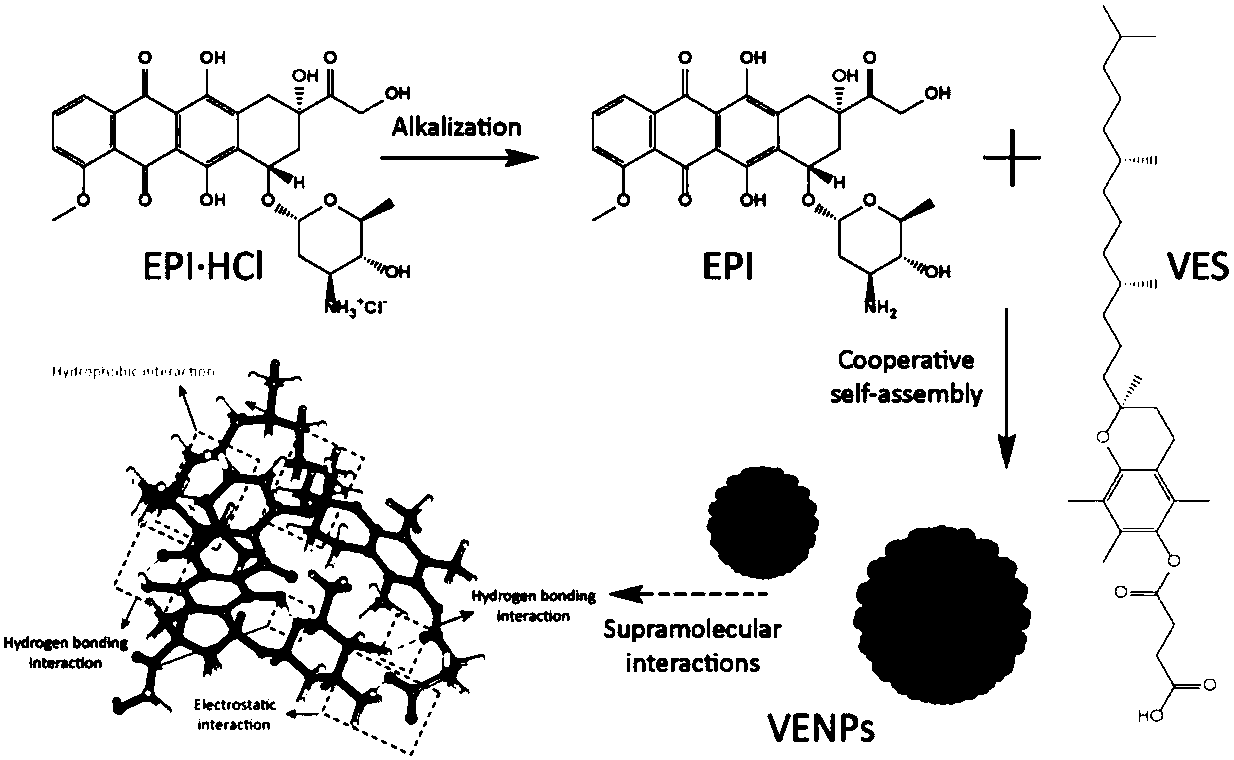
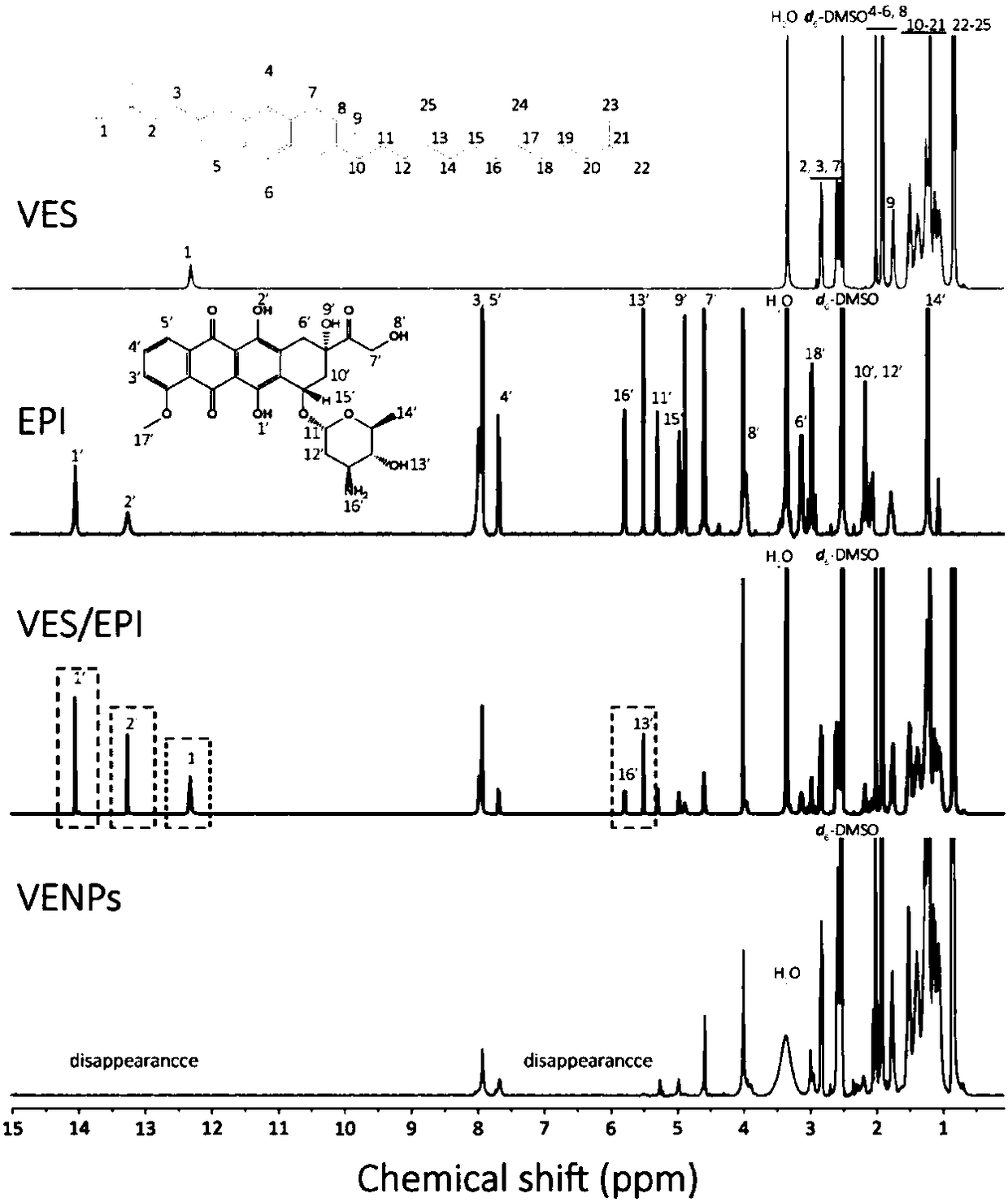
[0029] For an epirubicin VES complex in this embodiment, the mass ratio of epirubicin (or epirubicin hydrochloride) to VES (ie vitamin E succinate) is 1:1.
[0030] The preparation method of the above complex is as follows: 3 mg of epirubicin (or epirubicin hydrochloride) and 3 mg of VES are dissolved in 10 mL of dimethylformamide, and stirred and mixed for 12 hours at 20 ° C to obtain epirubicin The organic solution of mycin VES complex; remove the organic solution to obtain the epirubicin VES complex;
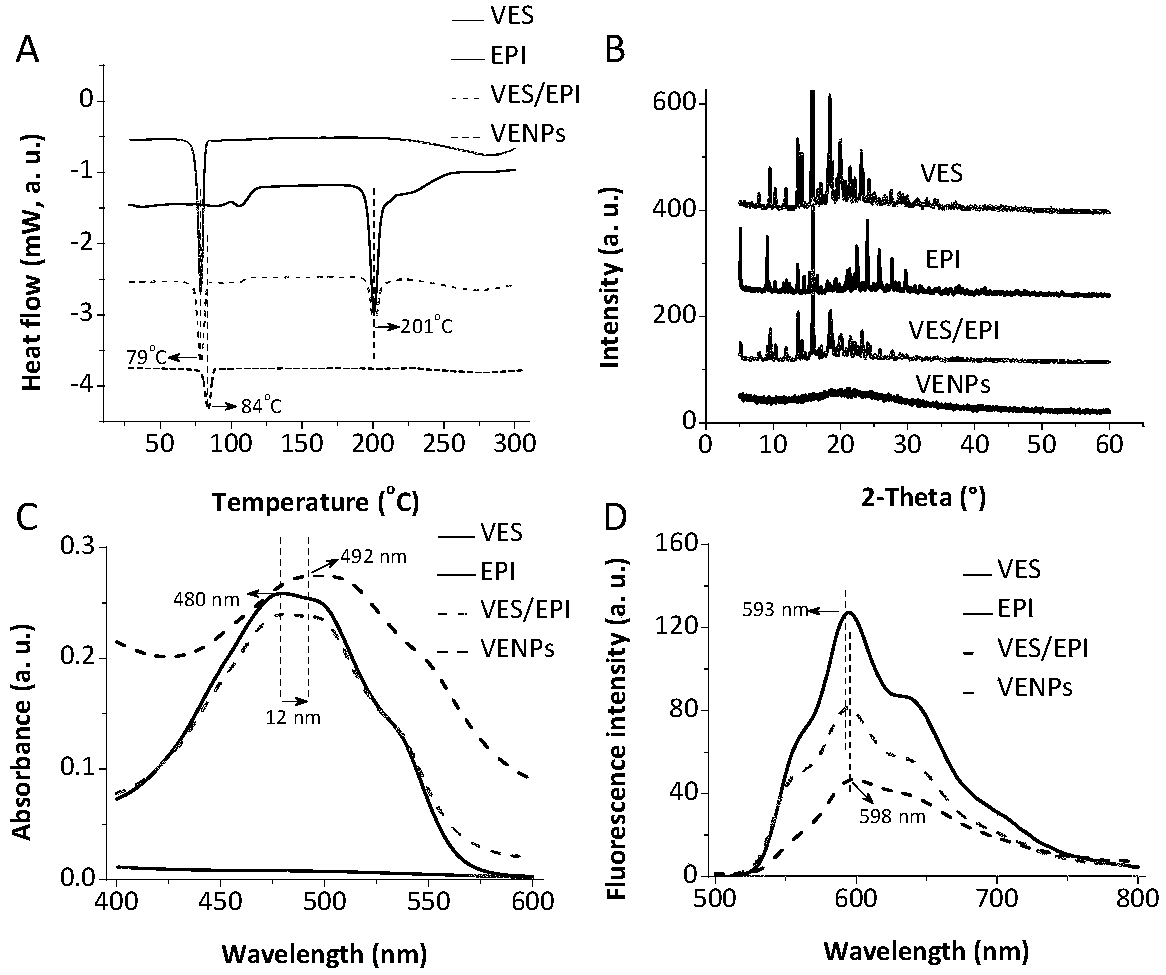
[0031] A preparation method of epirubicin VES composite nanoparticles is as follows: the above-mentioned composite organic solvent (or composite) is dialyzed with deionized water for 8 hours, the molecular weight cut-off of the dialysis bag used in the dialysis is 3500, and the organic solution is removed to obtain epirubicin. Doxorubicin VES complex nanoparticles.
[0032] A preparation method of an epirubicin VES complex sustained-release preparation is as follows: adding ...
Embodiment 2
[0034] 3mg of epirubicin (or epirubicin hydrochloride) and 6mg of VES were dissolved in 10mL of dimethylformamide, stirred and mixed for 12 hours at 20°C to obtain an organic solution of epirubicin VES complex, Then dialyze with deionized water for 8 hours, the molecular weight cut-off of the dialysis bag used in the dialysis is 3500, and remove the organic solution to obtain epirubicin VES complex nanoparticles.
[0035] After that, poloxamer and sodium chloride are added to the epirubicin VES complex nanoparticle solution, and the epirubicin VES complex slow-release preparation is obtained after vacuum freeze-drying.
Embodiment 3
[0037] 3mg of epirubicin (or epirubicin hydrochloride) and 9mg of VES were dissolved in 10mL of dimethylformamide, stirred and mixed for 12 hours at 20°C to obtain an organic solution of epirubicin VES complex, Then dialyze with deionized water for 8 hours, the molecular weight cut-off of the dialysis bag used in the dialysis is 3500, and remove the organic solution to obtain epirubicin VES complex nanoparticles.
[0038] After that, poloxamer and sodium chloride are added to the epirubicin VES complex nanoparticles, and the epirubicin VES complex slow-release preparation is obtained after vacuum freeze-drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com