Orlistat and organic acid calcium eutectic crystal body and medicine composition containing eutectic crystal body
A technology of orlistat and organic acid calcium, which is applied in the field of medicine, can solve the problems of low melting point, achieve full dissolution, shorten drying time, and improve the effect of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Study on the preparation method of orlistat and calcium supplement organic acid calcium co-crystal
[0044] (1) Study on the preparation of orlistat and calcium gluconate co-crystal
[0045] Take type II orlistat 0.497g (1.003×10 -3 mol), placed in a 100mL three-neck flask in an ultrasonic water bath (40°C), slowly added methanol / water mixed solvent (30:1, v / v) dropwise under stirring until the solid was completely dissolved to obtain liquid A, Leave in an ultrasonic water bath (40° C.) with stirring. Another calcium gluconate monohydrate (molecular formula: Ca[C 6 h 11 o 7 ] 2 ·H 2 O, molecular weight 448.40) 4.483g (containing calcium ions 9.998×10 -3 mol, gluconate ion 19.996×10 -3 mol), placed in a 500mL three-neck flask in an ultrasonic water bath (40°C), slowly added methanol / water mixed solvent (30:1, v / v) dropwise under stirring until the solid was completely dissolved to obtain liquid B, Leave in an ultrasonic water bath (40° C.) with stirrin...
Embodiment 2
[0052] Example 2 Determination of Melting Point of Orlistat and Calcium Supplement Organic Acid Calcium Cocrystal
[0053] Orlistat-calcium gluconate co-crystal, orlistat-vitamin C calcium co-crystal, orlistat-citrate prepared respectively in Example 1 were processed according to the melting point automatic measuring instrument method recorded in the 2015 edition of "Chinese Pharmacopoeia". Calcium citrate co-crystal and orlistat calcium aspartate co-crystal were measured for melting point and melting range, and the results are shown in Table 1.
[0054] Table 1 Melting point determination results of orlistat and calcium supplement organic acid calcium cocrystal
[0055] Co-crystal former (CCF)
Orlistat: CCF (molecule ratio / molar ratio)
Eutectic melting point (°C)
Eutectic melting range (°C)
calcium gluconate
1:5
253.52
<1
vitamin c calcium
1:4
170.91
<1
calcium citrate
2:7
220.27
<1
calcium a...
Embodiment 3
[0058] Example 3 Determination of the Molecular Ratio / Molar Ratio of Orlistat and Cocrystal Former (CCF) in Orlistat and Calcium Supplement Organic Acid Calcium Cocrystal
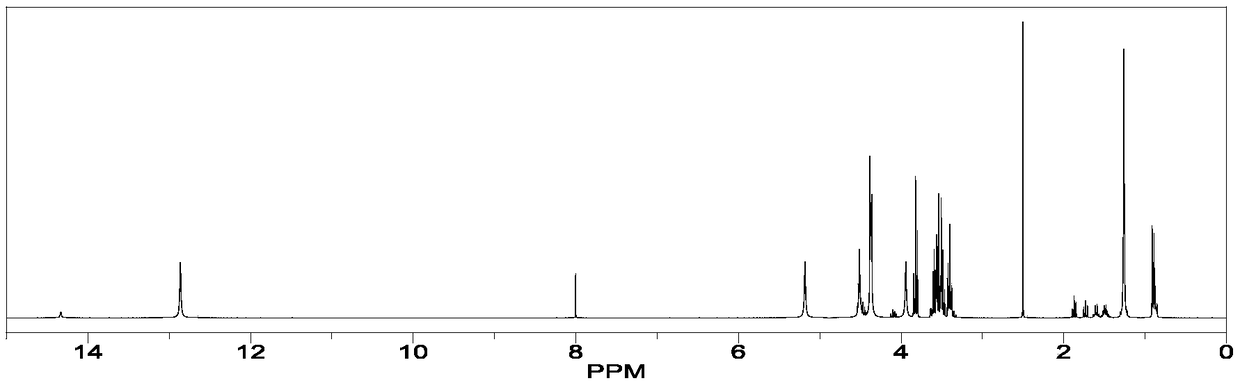
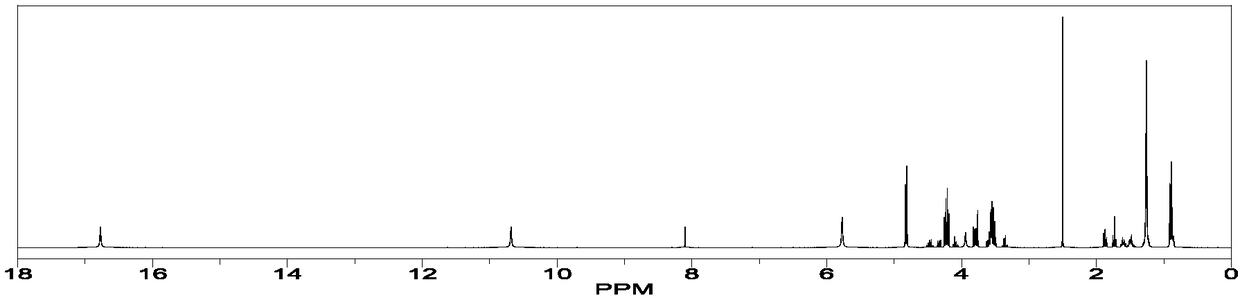
[0059] Using nuclear magnetic resonance ( 1 H-NMR, solution proton NMR) Determination of the molecular ratio / molar ratio of orlistat and co-crystal former (CFF) in orlistat and calcium supplement organic acid calcium co-crystal prepared in Example 1.
[0060] Method: Recorded on a Bruker-400MHz wavelength spectrometer equipped with an autosampler and controlled by a DRX400 console 1 H-NMR spectrum. Samples were dissolved in d6-DMSO for analysis. Data were acquired using standard Bruker load experiments with ICON-NMR v4.0.4 (construct 1) running with Topsin v1.3 (patch level 8).
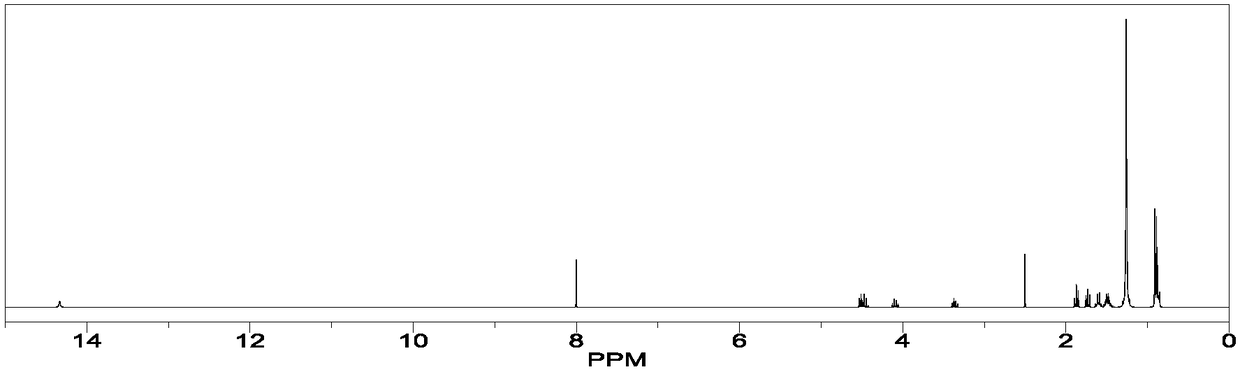
[0061] Result: orlistat 1 H-NMR spectrum such as figure 1As shown, the integrated areas of all peaks are 4.33 times of the integrated areas of the peaks in the range of 0.88-0.94 ppm.
[0062] Orlistat and calcium gluconate c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com