Chitinase inhibitor and application thereof
A technology of chitinase and inhibitors, applied in the direction of insecticides, anti-inflammatory agents, biocides, etc., can solve the problems of abnormal molting, death, and developmental stagnation of insects, and achieve a wide range of anti-allergic and asthma effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Taking chitinases SmChiA, SmChiB, AfChiB1, CeChtI, OvChtI, OfChtI, OfChtII-C2, OfChi-h, HsChit1 and AMCase as targets, 75 compounds listed in Table 1 and Table 2 were screened for inhibitors. Specific steps are as follows:
[0045] Positive control: Set up 3 parallel positive controls. Under the condition of 30℃ reaction temperature and 100μL reaction system, 2nmol / L chitinase and 50μmol / L substrate (MU-(GlcNAc) 2 ) was incubated in 20mmol / L pH 6.0 phosphate buffer for 30min, then 100μL 0.5mol / L sodium carbonate solution was added to terminate the reaction, and the reaction solution was excited with 360nm wavelength excitation light to measure the absorbance value at 450nm wavelength.
[0046] Experimental group: set up 3 parallel experimental groups. Under the condition of 30℃ reaction temperature and 100μL reaction system, 2nmol / L chitinase and 50μmol / L substrate (MU-(GlcNAc) 2 ) and the compounds corresponding to the concentrations in Tables 1 and 2 were incubated...
Embodiment 2
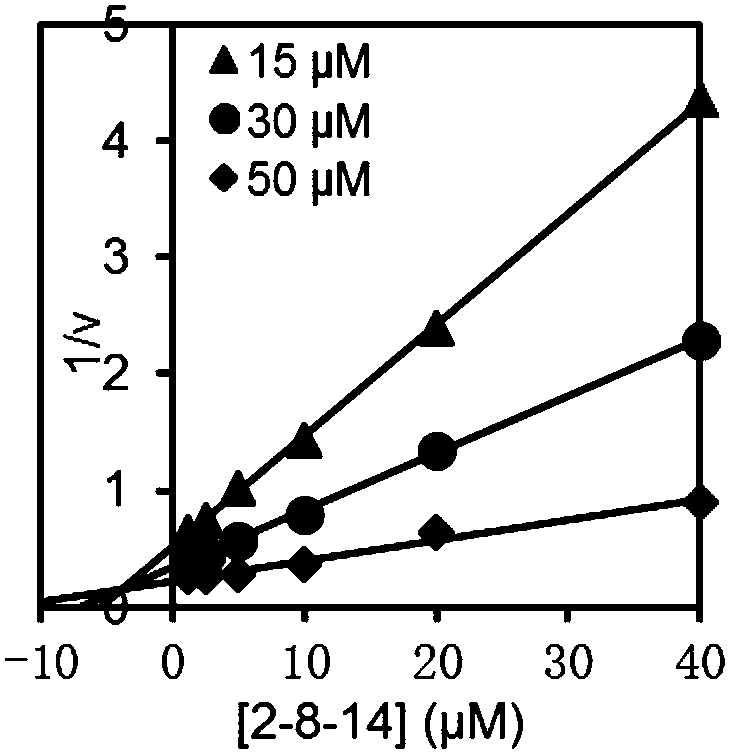
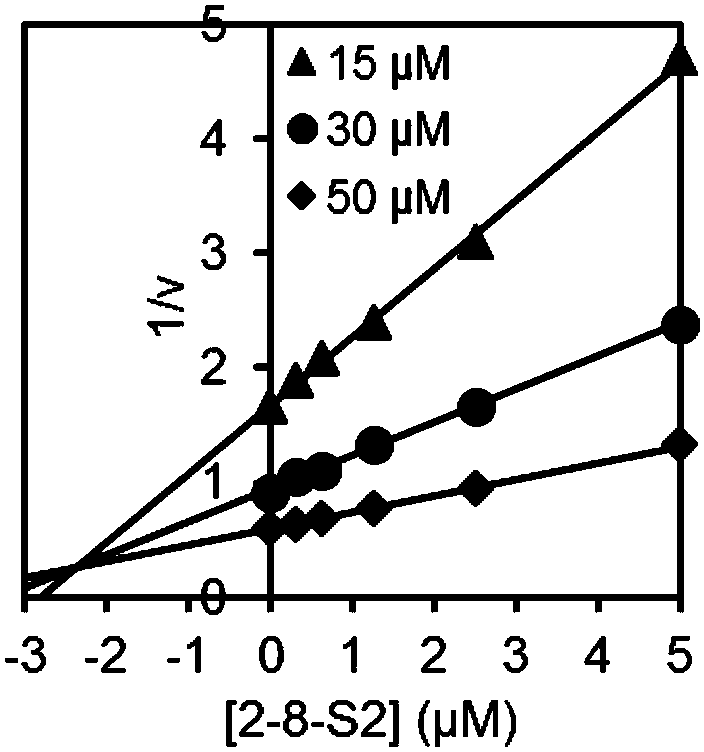
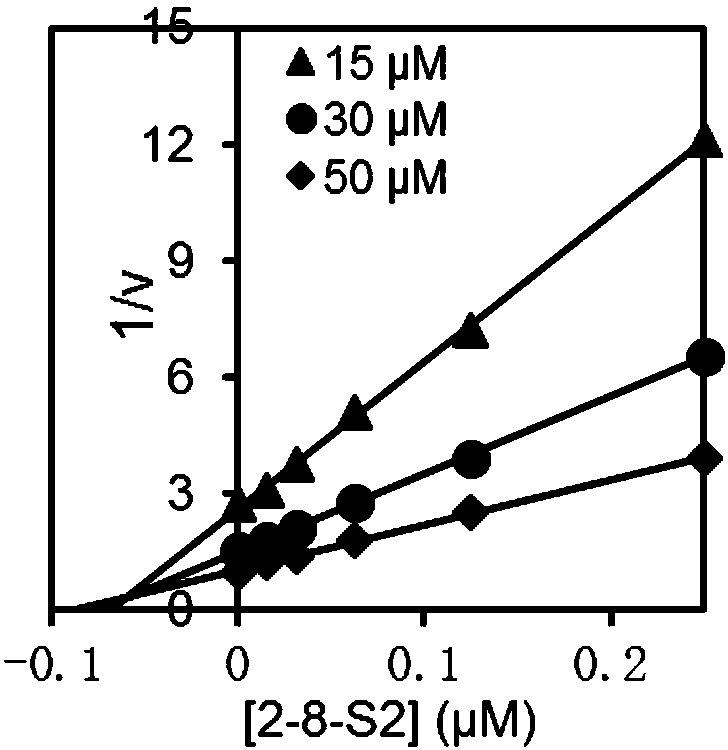
[0051] Inhibition constant K i determination
[0052] SmChiA, SmChiB, OfChi-h, HsChit1 and AMCase: MU-(GlcNAc) 2 As substrates, three groups of substrate concentration gradients were set up for the reaction, and the final concentrations were 15 μM, 30 μM and 50 μM, respectively. OvChtI: MU-(GlcNAc) 3 As substrates, three sets of substrate concentration gradients were set up for the reaction, and the final concentrations were 5 μM, 10 μM and 20 μM, respectively. Under each group of substrate concentrations, multiple groups of suitable compound concentration gradients were taken to measure the inhibitory activity. The reaction system was 100 μL, the buffer environment was 20 mM phosphate buffer, pH 6.0, the final enzyme concentration was 2 nM, the reaction temperature was 30 ° C, the reaction time was 30 min, and then 100 μL of 0.5 M sodium carbonate solution was added to terminate the reaction, and the released MU After being excited by 360nm excitation light, the absorbanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com