Monoclonal antibody against Napsin A protein, cell strain, preparation method and application
A monoclonal antibody and hybridoma cell line technology, applied in the field of biomedical engineering, can solve problems such as time-consuming, laborious, and difficult to type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Polypeptide Synthesis and Chemical Coupling with Carrier Protein
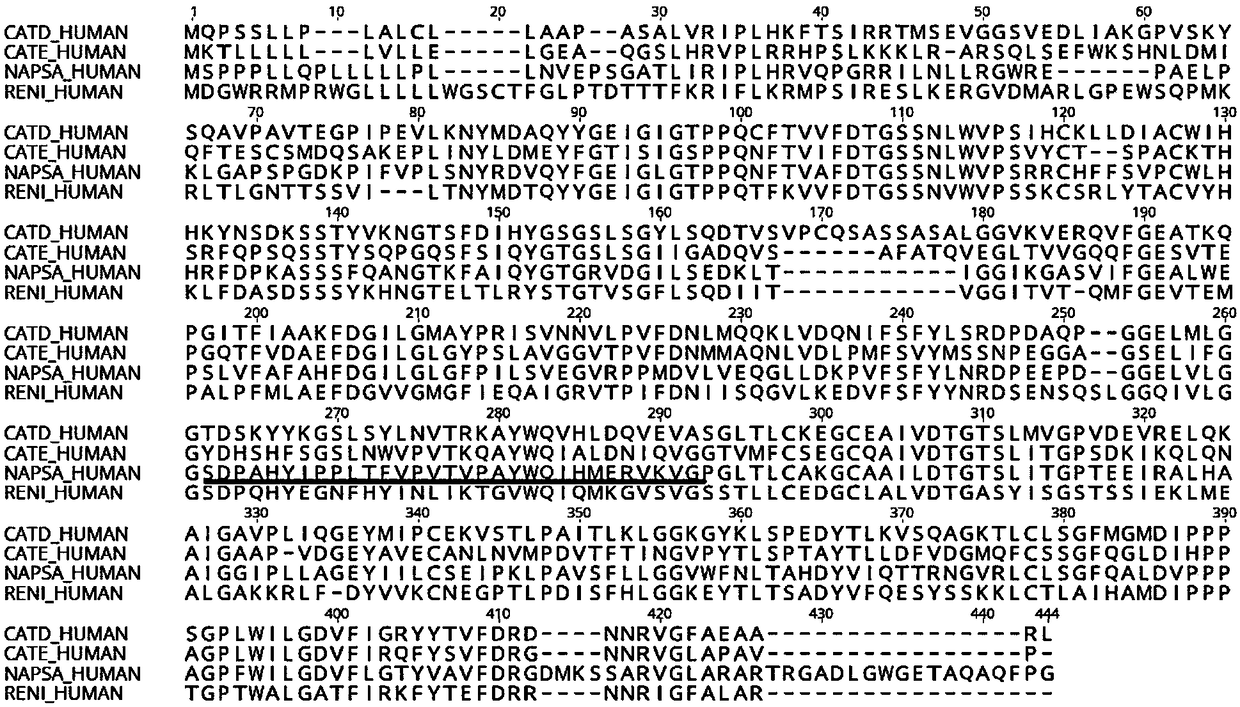
[0027] Select the Napsin A protein sequence numbered as P15391 as a standard sequence from the Uniprot database (http: / / www.uniprot.org), and its sequence is shown in the sequence table SEQID1, by BLAST (https: / / blast.ncbi.nlm. nih.gov / Blast.cgi) tool to compare its sequence differences with other proteins of the same family, and use the Protean module of DNASTAR8.0 software (www.dnastar.com) to conduct secondary structure, antigenicity and surface accessibility analysis.
[0028] The 238th to 268th amino acids of the protein were selected as the antigenic peptide, and a cysteine was added to the C-terminus of the sequence for coupling with the carrier protein KHL to obtain the immunogen. The immunogen consists of antigenic peptide, KLH carrier protein and the disulfide bond between them. The antigenic peptide is named Napsin A-PEP, and its sequence is shown as SEQID2 in the sequence listing...
Embodiment 2
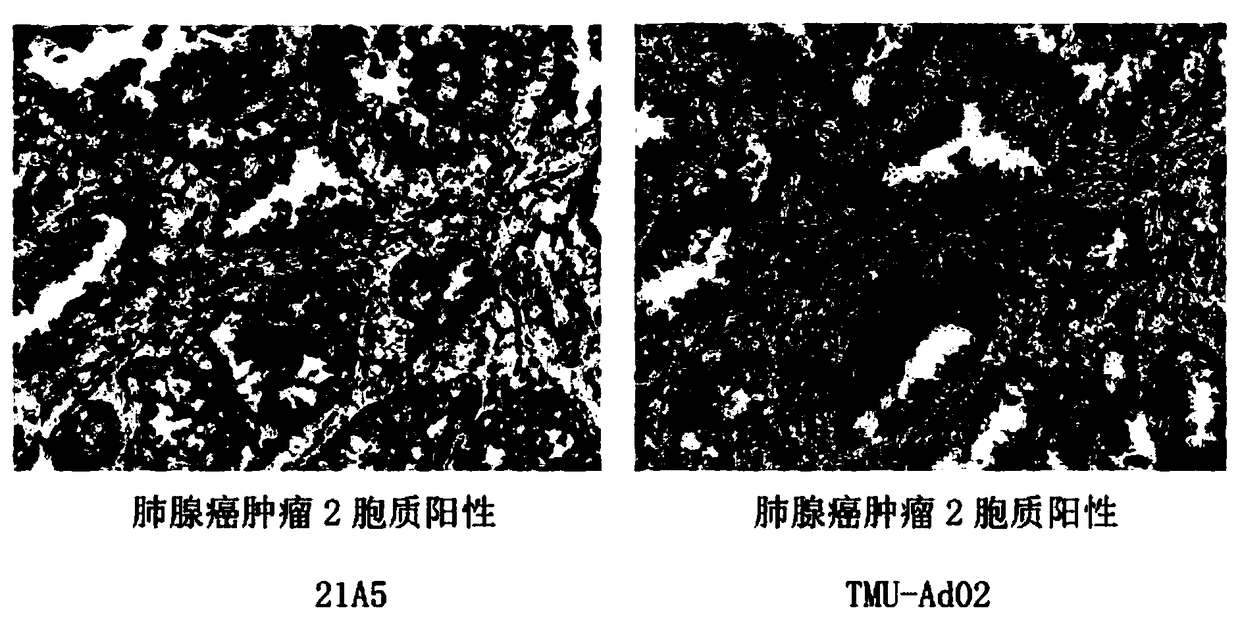
[0039] Example 2: Establishment of 21A5 hybridoma cell line
[0040] 1. Immunity
[0041] The immunogen obtained in Example 1 was emulsified with complete Freund's adjuvant (Sigma Company), and immunized with 4-6 week-old female Balb / c mice or ICR mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. ), the abdomen was subcutaneously injected into each mouse at 6 points, and the dose was 60 μg / only. Immunization was boosted every 14 days, and the antigen was emulsified with Freund's incomplete adjuvant (Sigma Company) at a dose of 30 μg per mouse. 7 days after the third booster immunization, indirect ELISA (wavelength 450nm) was used to detect the polyantibody titer of the anti-immunogen in the mouse serum. 50μg / only.
[0042] 2. Cell Fusion
[0043] Aseptically prepare the mouse splenocyte suspension that meets the immune standard, mix it with mouse myeloma cell sp2 / 0 (ATCC) at a ratio of 5:1, and centrifuge at 1500 rpm for 5 min. After the ...
Embodiment 3
[0048] Example 3 Preparation of Monoclonal Antibody by Ascites Induction Method
[0049] 1. Ascites preparation
[0050] The cells in the logarithmic growth phase were washed with serum-free medium and suspended, counted about 5×10 5 , 1ml. The suspended cells were injected intraperitoneally into mice previously sensitized with paraffin oil. Ascites collection was started 7 days later. The removed ascites was centrifuged at 4000rpm for 10min at 4°C. Carefully suck out the ascites in the middle and collect in a centrifuge tube, and store at 4°C or -20°C.
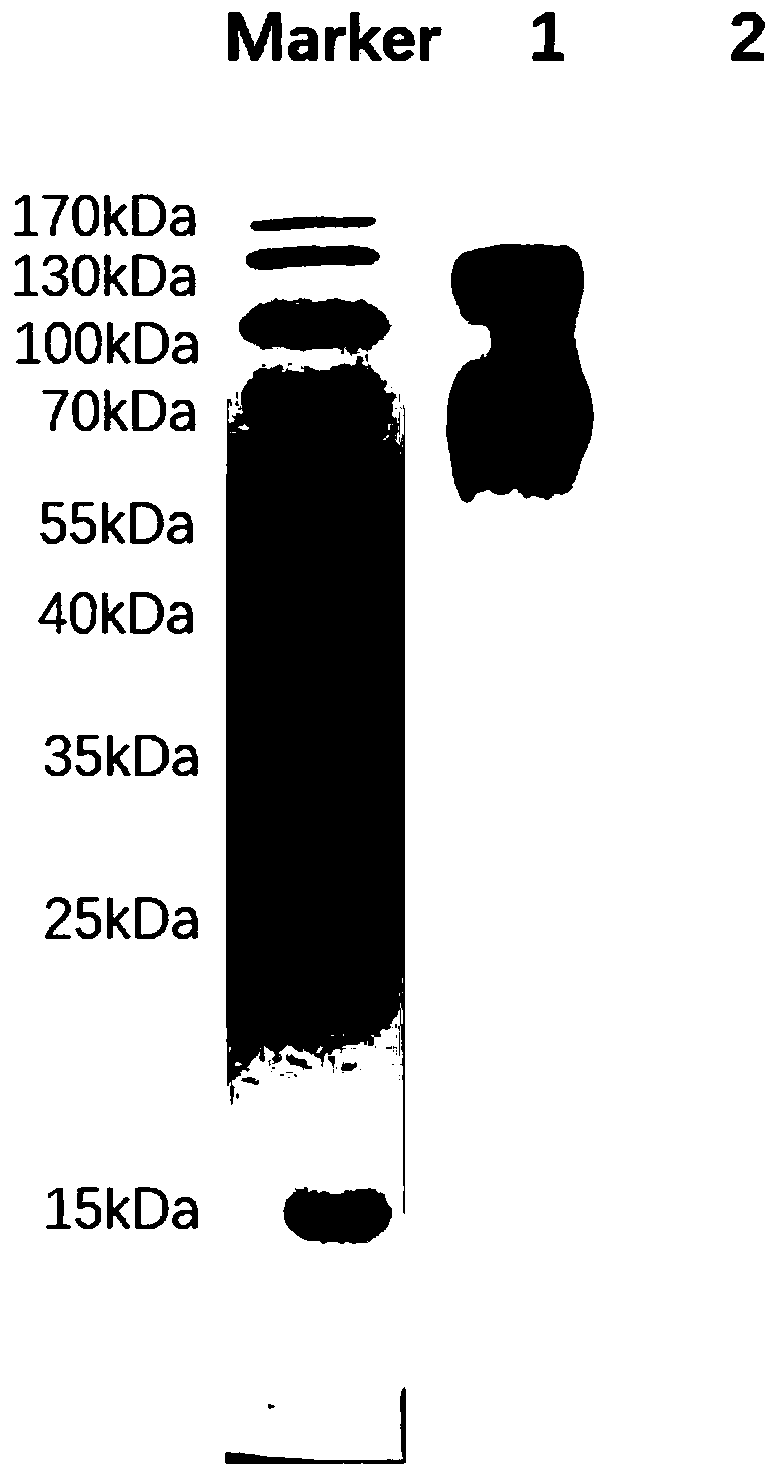
[0051] 2. Purification of monoclonal antibodies
[0052] Antibody was purified from ascitic fluid by HiTrap rProtein A FF (GE Company) affinity chromatography according to the manual. The purity was identified by SDS-PAGE gel, and the concentration was determined by Bradford method. Purified antibodies were stored at -20°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com