Compositions containing natural extracts and use thereof for skin and hair
A technology of composition and extract, applied in natural extract and pharmaceutical or cosmetic carrier, carrier or stabilization system, to reduce the graying process of mammalian hair, to reduce the appearance of non-pigmented skin areas in mammals, and to solve the problem of no Elderly people's nails and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
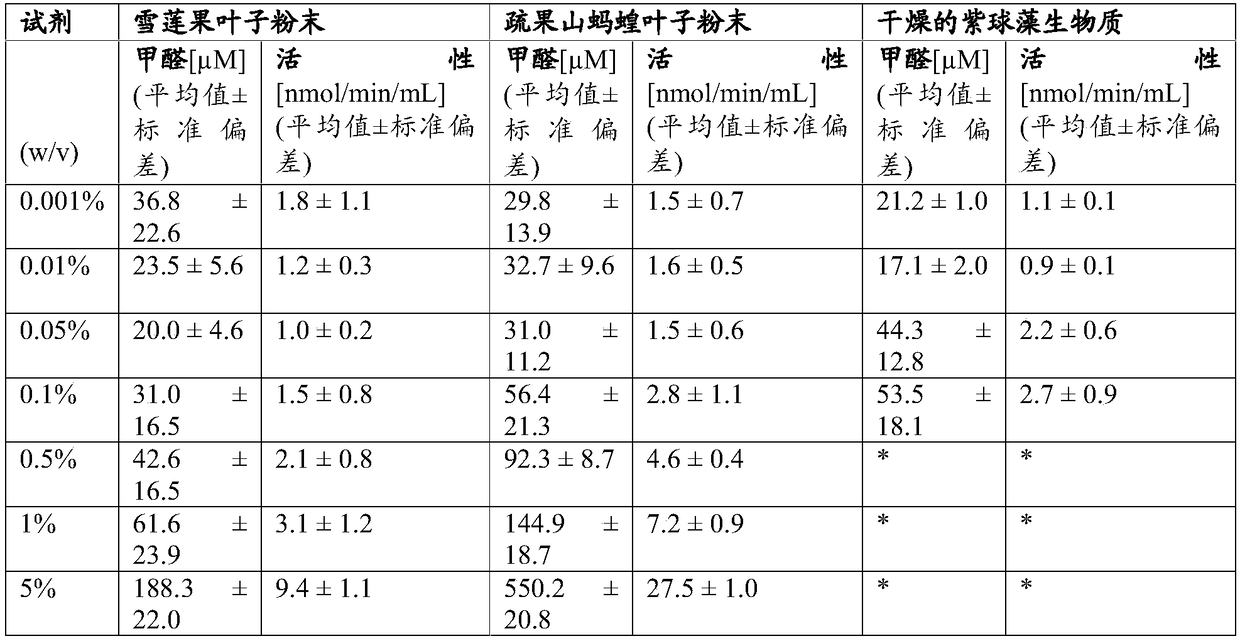
[0082] This study evaluated the hydrogen peroxide decomposition activity of the test agents. Catalase Assay Kit was obtained from Cayman Chemical Co. (Ann Arbor, MI). This assay measures hydrogen peroxide elimination activity (e.g. catalase activity) using Purpald (4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) as the chromogen in a colorimetric assay . The test involves a change in optical density (OD) at 540 nm, which is proportional to the effective activity present in the sample (based on reaction with methanol in the presence of hydrogen peroxide). Purpald, used as a chromogen, forms a bicyclic heterocycle with the resulting formaldehyde, which changes from colorless to purple after oxidation.
[0083] Native sample powders were suspended in water or PBS at 5% (w / v), sonicated to break down material into submicron particles, homogenized, and centrifuged at 1,000 RPM for 10 minutes to remove insoluble material. Serial dilutions were prepared for each test material and m...
Embodiment 2
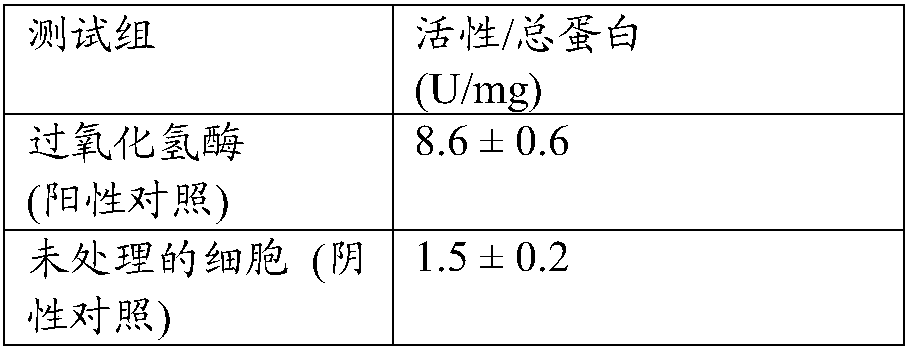
[0092] This study evaluated the effect of the test material on the endogenous cellular hydrogen peroxide degradation and elimination activity of cultured normal human keratinocytes. Hydrogen peroxide degradation was measured using protein lysates of treated cells using Purpald (4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) as the chromogen in a colorimetric assay active. The test involves a change in optical density (OD) at 540 nm that is proportional to the effective activity (based on reaction with methanol in the presence of hydrogen peroxide) present in the sample. Purpald, used as a chromogen, forms a bicyclic heterocycle with the resulting formaldehyde, which changes from colorless to purple after oxidation.
[0093]Sample powders of native test reagents were suspended in phosphate buffered saline (1xPBS) at 5% w / v and homogenized by sonication. After spinning at 1000 RPM for 10 min, the supernatant was sterilized by filtration using a 0.2 μm syringe filter.
[0094]...
Embodiment 3
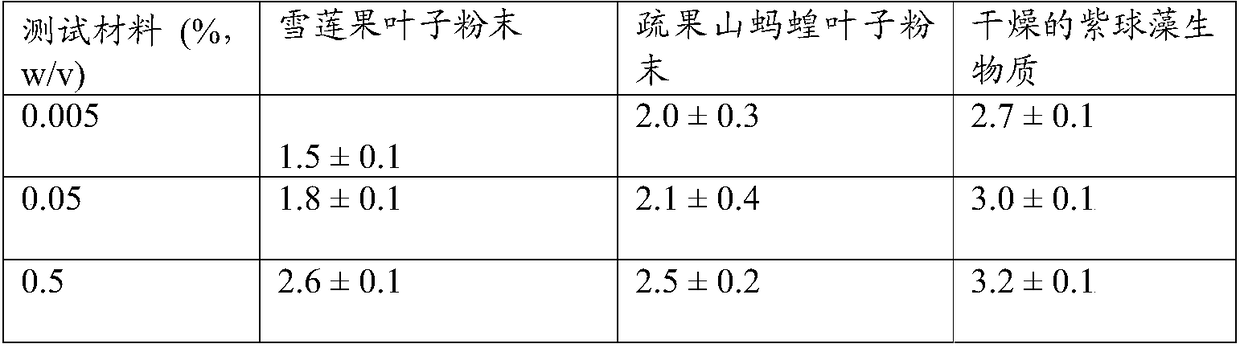
[0104] The aim of this study was to evaluate the intracellular antioxidant activity of the test material in cultured human epidermal keratinocytes in response to hydrogen peroxide injury. Cells were treated with test material, labeled with H2DCFDA and subsequently exposed to hydrogen peroxide. Relative fluorescence was measured as an indication of intracellular reactive oxygen species (ROS). The test involves a change in fluorescence that is directly proportional to the effective intracellular antioxidant activity compared to untreated and hydrogen peroxide-only treated samples.
[0105] Test reagents were stored at room temperature until use. Suspend the sample powder in phosphate buffered saline (1xPBS) to prepare a 10 mg / mL (1% w / v) stock solution, vortex for 1 min, and sonicate at 30-50% output for 10 min on ice at 4 °C Centrifuge at 1000 rpm for 10 minutes and sterilize the supernatant with a 0.2 μm syringe filter. Prepare this homogenized solution for each test materi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com